Development and organization of ocular dominance bands in primary visual cortex of the sable ferret
- PMID: 10213088
- PMCID: PMC2453001
Development and organization of ocular dominance bands in primary visual cortex of the sable ferret
Abstract
Thalamocortical afferents in the visual cortex of the adult sable ferret are segregated into eye-specific ocular dominance bands. The development of ocular dominance bands was studied by transneuronal labeling of the visual cortices of ferret kits between the ages of postnatal day 28 (P28) and P81 after intravitreous injections of either tritiated proline or wheat germ agglutinin-horseradish peroxidase. Laminar specificity was evident in the youngest animals studied and was similar to that in the adult by P50. In P28 and P30 ferret kits, no modulation reminiscent of ocular dominance bands was detectable in the pattern of labeling along layer IV. By P37 a slight fluctuation in the density of labeling in layer IV was evident in serial reconstructions. By P50, the amplitude of modulation had increased considerably but the pattern of ocular dominance bands did not yet appear mature. The pattern and degree of modulation of the ocular dominance bands resembled that in adult animals by P63. Flat mounts of cortex and serial reconstructions of layer IV revealed an unusual arrangement of inputs serving the two eyes in the region rostral to the periodic ocular dominance bands. In this region, inputs serving the contralateral eye were commonly fused along a mediolateral axis, rostral to which were large and sometimes fused patches of ipsilateral input.
Figures


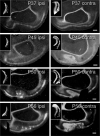



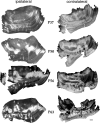


Similar articles
-
Nasotemporal asymmetries in V1: ocular dominance columns of infant, adult, and strabismic macaque monkeys.J Comp Neurol. 1997 Nov 10;388(1):32-46. doi: 10.1002/(sici)1096-9861(19971110)388:1<32::aid-cne3>3.0.co;2-p. J Comp Neurol. 1997. PMID: 9364237
-
The overall pattern of ocular dominance bands in cat visual cortex.J Neurosci. 1988 Jun;8(6):2183-200. doi: 10.1523/JNEUROSCI.08-06-02183.1988. J Neurosci. 1988. PMID: 3385494 Free PMC article.
-
An adult-like pattern of ocular dominance columns in striate cortex of newborn monkeys prior to visual experience.J Neurosci. 1996 Mar 1;16(5):1791-807. doi: 10.1523/JNEUROSCI.16-05-01791.1996. J Neurosci. 1996. PMID: 8774447 Free PMC article.
-
Ocular integration in the human visual cortex.Can J Ophthalmol. 2006 Oct;41(5):584-93. doi: 10.1016/S0008-4182(06)80027-X. Can J Ophthalmol. 2006. PMID: 17016529 Review.
-
Development of cortical circuits: lessons from ocular dominance columns.Nat Rev Neurosci. 2002 Jan;3(1):34-42. doi: 10.1038/nrn703. Nat Rev Neurosci. 2002. PMID: 11823803 Review.
Cited by
-
Early valproic acid exposure alters functional organization in the primary visual cortex.Exp Neurol. 2011 Mar;228(1):138-48. doi: 10.1016/j.expneurol.2010.12.025. Epub 2011 Jan 6. Exp Neurol. 2011. PMID: 21215743 Free PMC article.
-
Mechanisms underlying development of visual maps and receptive fields.Annu Rev Neurosci. 2008;31:479-509. doi: 10.1146/annurev.neuro.31.060407.125533. Annu Rev Neurosci. 2008. PMID: 18558864 Free PMC article. Review.
-
Thalamocortical Circuits and Functional Architecture.Annu Rev Vis Sci. 2018 Sep 15;4:263-285. doi: 10.1146/annurev-vision-091517-034122. Epub 2018 Jun 1. Annu Rev Vis Sci. 2018. PMID: 29856937 Free PMC article. Review.
-
Emergence of ocular dominance columns in cat visual cortex by 2 weeks of age.J Comp Neurol. 2001 Feb 5;430(2):235-49. doi: 10.1002/1096-9861(20010205)430:2<235::aid-cne1028>3.0.co;2-p. J Comp Neurol. 2001. PMID: 11135259 Free PMC article.
-
Deafferentation-induced plasticity of visual callosal connections: predicting critical periods and analyzing cortical abnormalities using diffusion tensor imaging.Neural Plast. 2012;2012:250196. doi: 10.1155/2012/250196. Epub 2012 Nov 8. Neural Plast. 2012. PMID: 23213572 Free PMC article. Review.
References
-
- Ault SJ, Leventhal AG, Vitek DJ, Creel DJ. Abnormal ipsilateral visual field representation in areas 17 and 18 of hypopigmented cats. J Comp Neurol. 1995;354:181–192. - PubMed
-
- Baker GE, Thompson ID, Krug K, Smyth D, Tolhurst DJ. Spatial-frequency tuning and geniculocortical projections in the visual cortex (areas 17 and 18) of the pigmented ferret. Eur J Neurosci. 1998;10:2657–2668. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

