The subcellular localization of PBX1 and EXD proteins depends on nuclear import and export signals and is modulated by association with PREP1 and HTH
- PMID: 10215622
- PMCID: PMC316640
- DOI: 10.1101/gad.13.8.946
The subcellular localization of PBX1 and EXD proteins depends on nuclear import and export signals and is modulated by association with PREP1 and HTH
Abstract
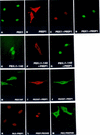
Nuclear localization of the Extradenticle (EXD) and PBX1 proteins is regionally restricted during Drosophila and mammalian development. We studied the subcellular localization of EXD, PBX, and their partners Homothorax (HTH) and PREP1, in different cell contexts. HTH and PREP1 are cytoplasmic and require association with EXD/PBX for nuclear localization. EXD and PBX1 are nuclear in murine fibroblasts but not in Drosophila Schneider cells, in which they are actively exported to the cytoplasm. Coexpression of EXD/PBX with HTH/PREP1 causes nuclear localization of their heterodimers in both cell contexts. We propose that heterodimerization with HTH/PREP induces nuclear translocation of EXD and PBX1 in specific cell contexts by blocking their nuclear export.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
