Arabidopsis STERILE APETALA, a multifunctional gene regulating inflorescence, flower, and ovule development
- PMID: 10215627
- PMCID: PMC316639
- DOI: 10.1101/gad.13.8.1002
Arabidopsis STERILE APETALA, a multifunctional gene regulating inflorescence, flower, and ovule development
Abstract
A recessive mutation in the Arabidopsis STERILE APETALA (SAP) causes severe aberrations in inflorescence and flower and ovule development. In sap flowers, sepals are carpelloid, petals are short and narrow or absent, and anthers are degenerated. Megasporogenesis, the process of meiotic divisions preceding the female gametophyte formation, is arrested in sap ovules during or just after the first meiotic division. More severe aberrations were observed in double mutants between sap and mutant alleles of the floral homeotic gene APETALA2 (AP2) suggesting that both genes are involved in the initiation of female gametophyte development. Together with the organ identity gene AGAMOUS (AG) SAP is required for the maintenance of floral identity acting in a manner similar to APETALA1. In contrast to the outer two floral organs in sap mutant flowers, normal sepals and petals develop in ag/sap double mutants, indicating that SAP negatively regulates AG expression in the perianth whorls. This supposed cadastral function of SAP is supported by in situ hybridization experiments showing ectopic expression of AG in the sap mutant. We have cloned the SAP gene by transposon tagging and revealed that it encodes a novel protein with sequence motifs, that are also present in plant and animal transcription regulators. Consistent with the mutant phenotype, SAP is expressed in inflorescence and floral meristems, floral organ primordia, and ovules. Taken together, we propose that SAP belongs to a new class of transcription regulators essential for a number of processes in Arabidopsis flower development.
Figures



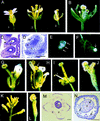
 ). The serine–glysine-rich domain is underlined. (*) The stop codon of the ORF. (C) Footprint alleles generated after excision of the I/dSpm element from the sap:I/dSpm allele. The phenotype is either as wild-type or sap mutant plants. The sequence of the duplications is underlined. In allele SAP-5.510 a duplication of four nucleotides results in the generation of a stopcodon indicated by an asterisks. In allele SAP-4.42 a duplication of two nucleotides (TA) is accompanied by a GC deletion, restoring the ORF.
). The serine–glysine-rich domain is underlined. (*) The stop codon of the ORF. (C) Footprint alleles generated after excision of the I/dSpm element from the sap:I/dSpm allele. The phenotype is either as wild-type or sap mutant plants. The sequence of the duplications is underlined. In allele SAP-5.510 a duplication of four nucleotides results in the generation of a stopcodon indicated by an asterisks. In allele SAP-4.42 a duplication of two nucleotides (TA) is accompanied by a GC deletion, restoring the ORF.
 ). The serine–glysine-rich domain is underlined. (*) The stop codon of the ORF. (C) Footprint alleles generated after excision of the I/dSpm element from the sap:I/dSpm allele. The phenotype is either as wild-type or sap mutant plants. The sequence of the duplications is underlined. In allele SAP-5.510 a duplication of four nucleotides results in the generation of a stopcodon indicated by an asterisks. In allele SAP-4.42 a duplication of two nucleotides (TA) is accompanied by a GC deletion, restoring the ORF.
). The serine–glysine-rich domain is underlined. (*) The stop codon of the ORF. (C) Footprint alleles generated after excision of the I/dSpm element from the sap:I/dSpm allele. The phenotype is either as wild-type or sap mutant plants. The sequence of the duplications is underlined. In allele SAP-5.510 a duplication of four nucleotides results in the generation of a stopcodon indicated by an asterisks. In allele SAP-4.42 a duplication of two nucleotides (TA) is accompanied by a GC deletion, restoring the ORF.

References
-
- Aarts MGM. ‘An En/Spm based transposable element system for gene isolation in Arabidopsis thaliana.’ PhD thesis. Wageningen, The Netherlands: Wageningen Agricultural University; 1996.
-
- Aarts MGM, Corzaan P, Stiekema WJ, Pereira A. A two-element Enhancer-Inhibitor transposon system in Arabidopsis thaliana. Mol Gen Genet. 1995;247:555–564. - PubMed
-
- Altschul SF, Giish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Angenent GC, Franken J, Busscher M, Colombo L, van Tunen AJ. Petal and stamen formation in petunia is regulated by the homeotic gene FBP1. Plant J. 1993;3:101–112. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
