TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling
- PMID: 10215628
- PMCID: PMC316636
- DOI: 10.1101/gad.13.8.1015
TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling
Abstract
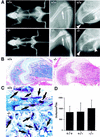
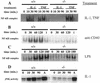
Bone resorption and remodeling is an intricately controlled, physiological process that requires the function of osteoclasts. The processes governing both the differentiation and activation of osteoclasts involve signals induced by osteoprotegerin ligand (OPGL), a member of tumor necrosis factor (TNF) superfamily, and its cognate receptor RANK. The molecular mechanisms of the intracellular signal transduction remain to be elucidated. Here we report that mice deficient in TNF receptor-associated factor 6 (TRAF6) are osteopetrotic with defects in bone remodeling and tooth eruption due to impaired osteoclast function. Using in vitro assays, we demonstrate that TRAF6 is crucial not only in IL-1 and CD40 signaling but also, surprisingly, in LPS signaling. Furthermore, like TRAF2 and TRAF3, TRAF6 is essential for perinatal and postnatal survival. These findings establish unexpectedly diverse and critical roles for TRAF6 in perinatal and postnatal survival, bone metabolism, LPS, and cytokine signaling.
Figures



References
-
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1 and IL-18-mediated function. Immunity. 1998;9:143–150. - PubMed
-
- Anderson DM, Maraskovsky E, Billingley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic cell function. Nature. 1997;390:175–179. - PubMed
-
- Arch RH, Gedrich RW, Thompson CB. Tumor necrosis factor receptor-associated factors (TRAFs)—a family of adapter proteins that regulates life and death. Genes & Dev. 1998;12:2821–2830. - PubMed
-
- Banchereau J, Bazan F, Blanchard F, Briere JP, Galizzi C, van Kooten C, Liu YL, Rousset F, Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. - PubMed
-
- Baron R, Revesloot J-H, Neff L, Chakraborty M, Chatterjee A, Lomri A, Horne W. Biology of the osteoclast. In: Noda M, editor. Cellular and molecular biology of bone. New York, NY: Springer Publishing; 1993. pp. 445–495.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
