The C-terminal fragment of the precursor tail lysozyme of bacteriophage T4 stays as a structural component of the baseplate after cleavage
- PMID: 10217762
- PMCID: PMC93713
- DOI: 10.1128/JB.181.9.2739-2744.1999
The C-terminal fragment of the precursor tail lysozyme of bacteriophage T4 stays as a structural component of the baseplate after cleavage
Abstract
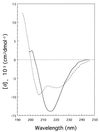
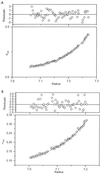
Tail-associated lysozyme of bacteriophage T4 (tail lysozyme), the product of gene 5 (gp 5), is an essential structural component of the hub of the phage baseplate. It is synthesized as a 63-kDa precursor, which later cleaves to form mature gp 5 with a molecular weight of 43,000. To elucidate the role of the C-terminal region of the precursor protein, gene 5 was cloned and overexpressed and the product was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, immunoblotting, analytical ultracentrifugation, and circular dichroism. It was shown that the precursor protein tends to be cleaved into two fragments during expression and that the cleavage site is close to or perhaps identical to the cleavage site in the infected cell. The two fragments, however, remained associated. The lysozyme activity of the precursor or the nicked protein is about 10% of that of mature gp 5. Both the N-terminal mature tail lysozyme and the C-terminal fragment were then isolated and characterized by far-UV circular dichroism and analytical ultracentrifugation. The latter remained trimeric after dissociation from the N-terminal fragment and is rich in beta-structure as predicted by an empirical method. To trace the fate of the C-terminal fragment, antiserum was raised against a synthesized peptide of the last 12 C-terminal residues. Surprisingly, the C-terminal fragment was found in the tail and the phage particle by immunoblotting. The significance of this finding is discussed in relation to the molecular assembly and infection process.
Figures



References
-
- Abedon S T. Lysis and the interaction between free phages and injected cells. In: Karam J D, editor. Molecular biology of bacteriophage T4. Washington, D.C: American Society for Microbiology; 1994. pp. 397–405.
-
- Bullock W O, Fernandez J M, Short J M. XL-1-Blue: a high efficiency plasmid transforming recA Escherichia coli strain with β-galactosidase selection. BioTechniques. 1987;5:376–379.
-
- Coombs D H, Arisaka F. T4 tail structure and function. In: Karam J D, editor. Molecular biology of bacteriophage T4. Washington, D.C: American Society for Microbiology; 1994. pp. 259–291.
-
- Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. pp. 471–510.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

