Ferric enterobactin binding and utilization by Neisseria gonorrhoeae
- PMID: 10217784
- PMCID: PMC93735
- DOI: 10.1128/JB.181.9.2895-2901.1999
Ferric enterobactin binding and utilization by Neisseria gonorrhoeae
Abstract
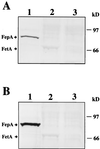
FetA, formerly designated FrpB, an iron-regulated, 76-kDa neisserial outer membrane protein, shows sequence homology to the TonB-dependent family of receptors that transport iron into gram-negative bacteria. Although FetA is commonly expressed by most neisserial strains and is a potential vaccine candidate for both Neisseria gonorrhoeae and Neisseria meningitidis, its function in cell physiology was previously undefined. We now report that FetA functions as an enterobactin receptor. N. gonorrhoeae FA1090 utilized ferric enterobactin as the sole iron source when supplied with ferric enterobactin at approximately 10 microM, but growth stimulation was abolished when an omega (Omega) cassette was inserted within fetA or when tonB was insertionally interrupted. FA1090 FetA specifically bound 59Fe-enterobactin, with a Kd of approximately 5 microM. Monoclonal antibodies raised against the Escherichia coli enterobactin receptor, FepA, recognized FetA in Western blots, and amino acid sequence comparisons revealed that residues previously implicated in ferric enterobactin binding by FepA were partially conserved in FetA. An open reading frame downstream of fetA, designated fetB, predicted a protein with sequence similarity to the family of periplasmic binding proteins necessary for transporting siderophores through the periplasmic space of gram-negative bacteria. An Omega insertion within fetB abolished ferric enterobactin utilization without causing a loss of ferric enterobactin binding. These data show that FetA is a functional homolog of FepA that binds ferric enterobactin and may be part of a system responsible for transporting the siderophore into the cell.
Figures





References
-
- Aebi C, Stone B, Beucher M, Cope L D, Maciver I, Thomas S E, McCracken G H, Jr, Sparling P F, Hansen E J. Expression of the CopB outer membrane protein by Moraxella catarrhalis is regulated by iron and affects iron acquisition from transferrin and lactoferrin. Infect Immun. 1996;64:2024–2030. - PMC - PubMed
-
- Beucher M. Ph.D. thesis. Chapel Hill: University of North Carolina; 1995.
-
- Biswas G D, Anderson J E, Sparling P F. Cloning and functional characterization of Neisseria gonorrhoeae tonB, exbB, and exbD genes. Mol Microbiol. 1997;24:169–179. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

