Motility of ActA protein-coated microspheres driven by actin polymerization
- PMID: 10220392
- PMCID: PMC21790
- DOI: 10.1073/pnas.96.9.4908
Motility of ActA protein-coated microspheres driven by actin polymerization
Abstract
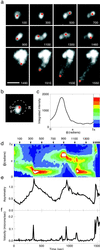
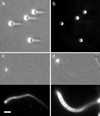
Actin polymerization is required for the generation of motile force at the leading edge of both lamellipodia and filopodia and also at the surface of motile intracellular bacterial pathogens such as Listeria monocytogenes. Local catalysis of actin filament polymerization is accomplished in L. monocytogenes by the bacterial protein ActA. Polystyrene beads coated with purified ActA protein can undergo directional movement in an actin-rich cytoplasmic extract. Thus, the actin polymerization-based motility generated by ActA can be used to move nonbiological cargo, as has been demonstrated for classical motor molecules such as kinesin and myosin. Initiation of unidirectional movement of a symmetrically coated particle is a function of bead size and surface protein density. Small beads (</=0.5 micrometer in diameter) initiate actin-based motility when local asymmetries are built up by random fluctuations of actin filament density or by thermal motion, demonstrating the inherent ability of the dynamic actin cytoskeleton to spontaneously self-organize into a polar structure capable of generating unidirectional force. Larger beads (up to 2 micrometers in diameter) can initiate movement only if surface asymmetry is introduced by coating the beads on one hemisphere. This explains why the relatively large L. monocytogenes requires polar distribution of ActA on its surface to move.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

