ADP-ribosylation factor 1 dependent clathrin-coat assembly on synthetic liposomes
- PMID: 10220410
- PMCID: PMC21808
- DOI: 10.1073/pnas.96.9.5013
ADP-ribosylation factor 1 dependent clathrin-coat assembly on synthetic liposomes
Abstract
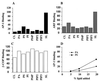
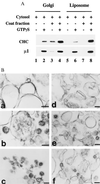
The assembly of clathrin-coated vesicles on Golgi membranes is initiated by the GTP-binding protein ADP ribosylation factor (ARF), which generates high-affinity membrane-binding sites for the heterotetrameric AP-1 adaptor complex. Once bound, the AP-1 recruits clathrin triskelia, which polymerize to form the coat. We have found that ARF.GTP also recruits AP-1 and clathrin onto protein-free liposomes. The efficiency of this process is modulated by the composition of the liposomes, with phosphatidylserine being the most stimulatory phospholipid. There is also a requirement for cytosolic factor(s) other than ARF. Thin-section electron microscopy shows the presence of clathrin-coated buds and vesicles that resemble those formed in vivo. These results indicate that AP-1-containing clathrin-coated vesicles can form in the absence of integral membrane proteins. Thus, ARF.GTP, appropriate lipids, and cytosolic factor(s) are the minimal components necessary for AP-1 clathrin-coat assembly.
Figures

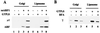
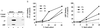

Similar articles
-
ADP-ribosylation factor 1 transiently activates high-affinity adaptor protein complex AP-1 binding sites on Golgi membranes.Mol Biol Cell. 1998 Jun;9(6):1323-37. doi: 10.1091/mbc.9.6.1323. Mol Biol Cell. 1998. PMID: 9614177 Free PMC article.
-
The assembly of AP-3 adaptor complex-containing clathrin-coated vesicles on synthetic liposomes.Mol Biol Cell. 2000 Nov;11(11):3723-36. doi: 10.1091/mbc.11.11.3723. Mol Biol Cell. 2000. PMID: 11071902 Free PMC article.
-
Adaptor protein 1-dependent clathrin coat assembly on synthetic liposomes and Golgi membranes.Methods Enzymol. 2001;329:379-87. doi: 10.1016/s0076-6879(01)29099-5. Methods Enzymol. 2001. PMID: 11210557 No abstract available.
-
Mechanisms of protein sorting and coat assembly: insights from the clathrin-coated vesicle pathway.Curr Opin Cell Biol. 1998 Aug;10(4):499-503. doi: 10.1016/s0955-0674(98)80065-3. Curr Opin Cell Biol. 1998. PMID: 9719871 Review.
-
Coat proteins regulating membrane traffic.Int Rev Cytol. 2000;195:67-144. doi: 10.1016/s0074-7696(08)62704-7. Int Rev Cytol. 2000. PMID: 10603575 Review.
Cited by
-
Gamma-BAR, a novel AP-1-interacting protein involved in post-Golgi trafficking.EMBO J. 2005 Mar 23;24(6):1122-33. doi: 10.1038/sj.emboj.7600600. Epub 2005 Mar 10. EMBO J. 2005. PMID: 15775984 Free PMC article.
-
Adaptor protein complexes and intracellular transport.Biosci Rep. 2014 Jul 29;34(4):e00123. doi: 10.1042/BSR20140069. Biosci Rep. 2014. PMID: 24975939 Free PMC article. Review.
-
Cigarette smoke inhibits engulfment of apoptotic cells by macrophages through inhibition of actin rearrangement.Am J Respir Cell Mol Biol. 2011 Apr;44(4):474-82. doi: 10.1165/rcmb.2009-0463OC. Epub 2010 Jun 4. Am J Respir Cell Mol Biol. 2011. PMID: 20525804 Free PMC article.
-
Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor.EMBO J. 2002 Sep 16;21(18):4915-26. doi: 10.1093/emboj/cdf487. EMBO J. 2002. PMID: 12234931 Free PMC article.
-
Membrane characteristics tune activities of endosomal and autophagic human VPS34 complexes.Elife. 2020 Jun 30;9:e58281. doi: 10.7554/eLife.58281. Elife. 2020. PMID: 32602837 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

