Prevention of experimental antiphospholipid syndrome and endothelial cell activation by synthetic peptides
- PMID: 10220436
- PMCID: PMC21834
- DOI: 10.1073/pnas.96.9.5164
Prevention of experimental antiphospholipid syndrome and endothelial cell activation by synthetic peptides
Abstract
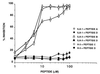
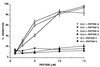
Antiphospholipid syndrome (APS) is characterized by recurrent fetal loss, repeated thromboembolic phenomena, and thrombocytopenia. The syndrome is believed to be caused by antiphospholipid beta-2-glycoprotein-I (beta2GPI)-dependent Abs or anti-beta2GPI Abs by themselves. Using a hexapeptide phage display library, we identified three hexapeptides that react specifically with the anti-beta2GPI mAbs ILA-1, ILA-3, and H-3, which cause endothelial cell activation and induce experimental APS. To enhance the binding of the peptides to the corresponding mAbs, the peptides were lengthened to correspond with the site of the beta2GPI epitope being recognized by these mAbs. As a result, the following three peptides were prepared: A, NTLKTPRVGGC, which binds to ILA-1 mAb; B, KDKATFGCHDGC, which binds to ILA-3 mAb; and C, CATLRVYKGG, which binds to H-3 mAb. Peptides A, B, and C specifically inhibit both in vitro and in vivo the biological functions of the corresponding anti-beta2GPI mAbs. Exposure of endothelial cells to anti-beta2GPI mAbs and their corresponding peptides led to the inhibition of endothelial cell activation, as shown by decreased expression of adhesion molecules (E-selectin, ICAM-1, VCAM-1) and monocyte adhesion. In vivo infusion of each of the anti-beta2GPI mAbs into BALB/c mice, followed by administration of the corresponding specific peptides, prevented the peptide-treated mice from developing experimental APS. The use of synthetic peptides that focus on neutralization of pathogenic anti-beta2GPI Abs represents a possible new therapeutic approach to APS.
Figures


References
-
- Hughes R V, Harris E N, Gharavi A E. J Rheumatol. 1986;13:486–492. - PubMed
-
- McNeil H P, Chesterman C N, Krilis S A. Adv Immunol. 1991;49:193–115. - PubMed
-
- Hughes G R V. Lancet. 1993;342:341–344. - PubMed
-
- Tsutsumi A, Matsuura E, Ichikawa K, Fujisaku A, Mukai M, Kobayashi S, Koike T. Arthritis Rheum. 1996;391:466–471. - PubMed
-
- Galli M, Comfurious P, Massen C, Hemker M H, Be-Bates P J, van-Breda-Vriesman P J. Lancet. 1992;335:1544–1546. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

