Clonal populations of hematopoietic cells with paroxysmal nocturnal hemoglobinuria genotype and phenotype are present in normal individuals
- PMID: 10220445
- PMCID: PMC21843
- DOI: 10.1073/pnas.96.9.5209
Clonal populations of hematopoietic cells with paroxysmal nocturnal hemoglobinuria genotype and phenotype are present in normal individuals
Abstract
In paroxysmal nocturnal hemoglobinuria (PNH), acquired somatic mutations in the PIG-A gene give rise to clonal populations of red blood cells unable to express proteins linked to the membrane by a glycosylphosphatidylinositol anchor. These proteins include the complement inhibitors CD55 and CD59, and this explains the hypersensitivity to complement of red cells in PNH patients, manifested by intravascular hemolysis. The factors that determine to what extent mutant clones expand have not yet been pinpointed; it has been suggested that existing PNH clones may have a conditional growth advantage depending on some factor (e.g., autoimmune) present in the marrow environment of PNH patients. Using flow cytometric analysis of granulocytes, we now have identified cells that have the PNH phenotype, at an average frequency of 22 per million (range 10-51 per million) in nine normal individuals. These rare cells were collected by flow sorting, and exons 2 and 6 of the PIG-A gene were amplified by nested PCR. We found PIG-A mutations in six cases: four missense, one frameshift, and one nonsense mutation. PNH red blood cells also were identified at a frequency of eight per million. Thus, small clones with PIG-A mutations exist commonly in normal individuals, showing clearly that PIG-A gene mutations are not sufficient for the development of PNH. Because PIG-A encodes an enzyme essential for the expression of a host of surface proteins, the PIG-A gene provides a highly sensitive system for the study of somatic mutations in hematopoietic cells.
Figures
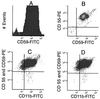


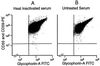

References
-
- Oni S-B, Osunkoya B-O, Luzzatto L. Blood. 1970;36:145–152. - PubMed
-
- Rosse W-F, Ware R-E. Blood. 1995;86:3277–3286. - PubMed
-
- Terstappen L-W, Nguyen M, Huang S, Lazarus H, Medof M-E. Br J Hematol. 1993;84:504–514. - PubMed
-
- Miyata T, Yamada N, Iida Y, Nishimura J, Takeda J, Kitani T, Kinoshita T. N Engl J Med. 1994;330:249–255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

