Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages
- PMID: 10220446
- PMCID: PMC21844
- DOI: 10.1073/pnas.96.9.5215
Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages
Abstract
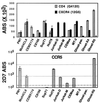
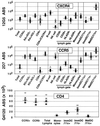
CCR5 and CXCR4 are the major HIV-1 coreceptors for R5 and X4 HIV-1 strains, respectively, and a threshold number of CD4 and chemokine receptor molecules is required to support virus infection. Therefore, we used a quantitative fluorescence-activated cell sorting assay to determine the number of CD4, CCR5, and CXCR4 antibody-binding sites (ABS) on various T cell lines, T cell subsets, peripheral blood dendritic cells (PBDC), and monocyte-derived macrophages by using four-color fluorescence-activated cell sorting analysis on fresh whole blood. Receptor levels varied dramatically among the various subsets examined and typically varied from 2- to 5-fold between individuals. CCR5 was expressed at much higher levels in CD4+/CD45RO+/CD62L-true memory cells compared with CD4+/CD45RO+/CD62L+ cells. Fresh PBDC had the highest number of CCR5 ABS among the leukocyte subsets examined but had few CXCR4 ABS, affording a strategy for sort-purifying PBDC. In vitro maturation of PBDC resulted in median 3- and 41-fold increases in CCR5 and CXCR4 ABS, respectively. We found that macrophage colony-stimulating factor caused the greatest up-regulation of both CCR5 and CXCR4 on macrophage maturation (from approximately 5,000 to approximately 50, 000 ABS) whereas granulocyte-macrophage colony-stimulating factor caused a marked decrease of CXCR4 (from approximately 5,000 ABS to <500) while up-regulating CCR5 expression (from approximately 5,000 to approximately 20,000 ABS). Absolute ABS for CD4 and the major HIV-1 coreceptors serve as a more quantitative measure of cell surface expression, and we propose that this be used for future studies looking at the modulation of CD4 or chemokine receptor expression by cytokines, HIV-1 infection, or receptor polymorphisms.
Figures

References
-
- Bieniasz P D, Cullen B R. Front Biosci. 1998;3:44–58. - PubMed
-
- Doms R W, Peiper S C. Virology. 1997;235:179–190. - PubMed
-
- Moore J P, Trkola A, Dragic T. Curr Opin Immunol. 1997;9:551–562. - PubMed
-
- Winkler C, Modi W, Smith M W, Nelson G W, Wu X, Carrington M, Dean M, Honjo T, Tashiro K, Yabe D, et al. Science. 1998;279:389–393. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

