The first-nucleotide binding domain of the cystic-fibrosis transmembrane conductance regulator is important for inhibition of the epithelial Na+ channel
- PMID: 10220462
- PMCID: PMC21860
- DOI: 10.1073/pnas.96.9.5310
The first-nucleotide binding domain of the cystic-fibrosis transmembrane conductance regulator is important for inhibition of the epithelial Na+ channel
Abstract
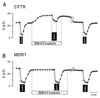
The cystic-fibrosis transmembrane conductance regulator (CFTR) functions as a cAMP-regulated Cl- channel and as a regulator of other membrane conductances. cAMP-dependent activation of CFTR inhibits epithelial Na+ channels (ENaC). The specificity of interaction between CFTR and ENaC was examined by coexpression of ENaC and ATP-binding cassette (ABC) proteins other than CFTR. In addition, we identified domains within CFTR that are of particular importance for the inhibition of ENaC. To that end, two-electrode voltage-clamp experiments were performed on Xenopus oocytes coexpressing ENaC together with CFTR, the multidrug resistance protein MDR1, the sulfonyl urea receptor SUR1, or the cadmium permease YCF1. Except for CFTR, none of the other ABC proteins were able to inhibit ENaC. Several truncated versions of CFTR were examined for their inhibitory effects on ENaC. In fact, it is shown that C-terminal truncated CFTR is able to inhibit ENaC on activation by intracellular cAMP. Moreover, the data also show that an intact first-nucleotide binding domain (NBF-1) is important for inhibition of ENaC. We conclude that NBF-1 of CFTR contains a CFTR-specific regulatory site that down-regulates ENaC. It is speculated that this regulatory site also is needed for CFTR-mediated interactions with other membrane proteins and that it is not present in NBF-1 of other ABC proteins.
Figures

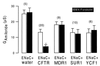
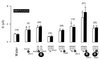


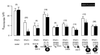
Similar articles
-
Sulfonylurea receptors inhibit the epithelial sodium channel (ENaC) by reducing surface expression.Pflugers Arch. 2001 Aug;442(5):752-61. doi: 10.1007/s004240100597. Pflugers Arch. 2001. PMID: 11512032
-
Cystic fibrosis transmembrane conductance regulator-dependent up-regulation of Kir1.1 (ROMK) renal K+ channels by the epithelial sodium channel.J Biol Chem. 2002 Jul 12;277(28):25377-84. doi: 10.1074/jbc.M201925200. Epub 2002 May 6. J Biol Chem. 2002. PMID: 11994290
-
Cl- interference with the epithelial Na+ channel ENaC.J Biol Chem. 2005 Sep 9;280(36):31587-94. doi: 10.1074/jbc.M504347200. Epub 2005 Jul 18. J Biol Chem. 2005. PMID: 16027156
-
ENaC is inhibited by an increase in the intracellular Cl(-) concentration mediated through activation of Cl(-) channels.Pflugers Arch. 2003 Jan;445(4):504-12. doi: 10.1007/s00424-002-0958-y. Epub 2002 Nov 20. Pflugers Arch. 2003. PMID: 12548397 Review.
-
CFTR is a conductance regulator as well as a chloride channel.Physiol Rev. 1999 Jan;79(1 Suppl):S145-66. doi: 10.1152/physrev.1999.79.1.S145. Physiol Rev. 1999. PMID: 9922379 Review.
Cited by
-
Electrophysiological evidence for the presence of cystic fibrosis transmembrane conductance regulator (CFTR) in mouse sperm.J Cell Physiol. 2013 Mar;228(3):590-601. doi: 10.1002/jcp.24166. J Cell Physiol. 2013. PMID: 22833409 Free PMC article.
-
CFTR fails to inhibit the epithelial sodium channel ENaC expressed in Xenopus laevis oocytes.J Physiol. 2005 May 1;564(Pt 3):671-82. doi: 10.1113/jphysiol.2004.079046. Epub 2005 Mar 3. J Physiol. 2005. PMID: 15746174 Free PMC article.
-
Impaired Regulatory Volume Decrease and Characterization of Underlying Volume-Activated Currents in Cystic Fibrosis Human Cholangiocyte Cell Line.J Membr Biol. 2022 Jun;255(2-3):261-276. doi: 10.1007/s00232-022-00216-2. Epub 2022 Jan 30. J Membr Biol. 2022. PMID: 35098342
-
Lung infections associated with cystic fibrosis.Clin Microbiol Rev. 2002 Apr;15(2):194-222. doi: 10.1128/CMR.15.2.194-222.2002. Clin Microbiol Rev. 2002. PMID: 11932230 Free PMC article. Review.
-
Does epithelial sodium channel hyperactivity contribute to cystic fibrosis lung disease?J Physiol. 2013 Sep 15;591(18):4377-87. doi: 10.1113/jphysiol.2012.240861. Epub 2013 Jul 22. J Physiol. 2013. PMID: 23878362 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

