Chaperonin function: folding by forced unfolding
- PMID: 10221918
- PMCID: PMC3427652
- DOI: 10.1126/science.284.5415.822
Chaperonin function: folding by forced unfolding
Abstract
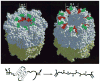
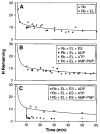
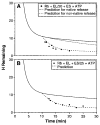
The ability of the GroEL chaperonin to unfold a protein trapped in a misfolded condition was detected and studied by hydrogen exchange. The GroEL-induced unfolding of its substrate protein is only partial, requires the complete chaperonin system, and is accomplished within the 13 seconds required for a single system turnover. The binding of nucleoside triphosphate provides the energy for a single unfolding event; multiple turnovers require adenosine triphosphate hydrolysis. The substrate protein is released on each turnover even if it has not yet refolded to the native state. These results suggest that GroEL helps partly folded but blocked proteins to fold by causing them first to partially unfold. The structure of GroEL seems well suited to generate the nonspecific mechanical stretching force required for forceful protein unfolding.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

