Contribution of methanotrophic and nitrifying bacteria to CH4 and NH4+ oxidation in the rhizosphere of rice plants as determined by new methods of discrimination
- PMID: 10223965
- PMCID: PMC91262
- DOI: 10.1128/AEM.65.5.1826-1833.1999
Contribution of methanotrophic and nitrifying bacteria to CH4 and NH4+ oxidation in the rhizosphere of rice plants as determined by new methods of discrimination
Abstract
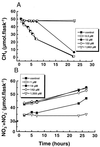
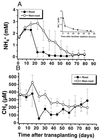
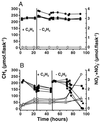
Methanotrophic and nitrifying bacteria are both able to oxidize CH4 as well as NH4+. To date it is not possible to estimate the relative contribution of methanotrophs to nitrification and that of nitrifiers to CH4 oxidation and thus to assess their roles in N and C cycling in soils and sediments. This study presents new options for discrimination between the activities of methanotrophs and nitrifiers, based on the competitive inhibitor CH3F and on recovery after inhibition with C2H2. By using rice plant soil as a model system, it was possible to selectively inactivate methanotrophs in soil slurries at a CH4/CH3F/NH4+ molar ratio of 0.1:1:18. This ratio of CH3F to NH4+ did not affect ammonia oxidation, but methane oxidation was inhibited completely. By using the same model system, it could be shown that after 24 h of exposure to C2H2 (1,000 parts per million volume), methanotrophs recovered within 24 h while nitrifiers stayed inactive for at least 3 days. This gave an "assay window" of 48 h when only methanotrophs were active. Applying both assays to model microcosms planted with rice plants demonstrated a major contribution of methanotrophs to nitrification in the rhizosphere, while the contribution of nitrifiers to CH4 oxidation was insignificant.
Figures



References
-
- Armstrong W, Brändle R, Jackson M B. Mechanisms of flood tolerance in plants. Acta Bot Neerl. 1994;43:307–358.
-
- Arth I, Frenzel P, Conrad R. Denitrification coupled to nitrification in the rhizosphere of rice. Soil Biol Biochem. 1998;30:509–515.
-
- Arth, I. 1998. Personal communication.
LinkOut - more resources
Full Text Sources

