Study of the response of a biofilm bacterial community to UV radiation
- PMID: 10223995
- PMCID: PMC91292
- DOI: 10.1128/AEM.65.5.2025-2031.1999
Study of the response of a biofilm bacterial community to UV radiation
Abstract
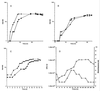
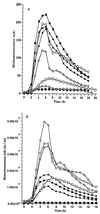
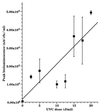
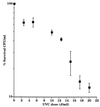
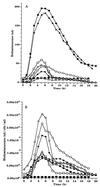
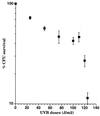
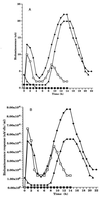
We have developed a bioluminescent whole-cell biosensor that can be incorporated into biofilm ecosystems. RM4440 is a Pseudomonas aeruginosa FRD1 derivative that carries a plasmid-based recA-luxCDABE fusion. We immobilized RM4440 in an alginate matrix to simulate a biofilm, and we studied its response to UV radiation damage. The biofilm showed a protective property by physical shielding against UV C, UV B, and UV A. Absorption of UV light by the alginate matrix translated into a higher survival rate than observed with planktonic cells at similar input fluences. UV A was shown to be effectively blocked by the biofilm matrix and to have no detectable effects on cells contained in the biofilm. However, in the presence of photosensitizers (i.e., psoralen), UV A was effective in inducing light production and cell death. RM4440 has proved to be a useful tool to study microbial communities in a noninvasive manner.
Figures


References
-
- Averbeck D. Recent advances in psoralen phototoxicity mechanism. Photochem Photobiol. 1989;50:859–882. - PubMed
-
- Bauluz C, Paramio J M, de Vidania R. Further studies on the lethal and mutagenic effects of 8-methoxypsoralen-induced lesions on plasmid DNA. Cell Mol Biol. 1991;37:481–500. - PubMed
-
- Booth M G. Solar ultraviolet radiation and the role of RecA in marine bacteria. Ph.D. dissertation. Stillwater: Oklahoma State University; 1997.
-
- Christensen B E, Characklis W G. Physical and chemical properties of biofilms. In: Characklis W G, Marshall K C, editors. Biofilms. New York, N.Y: John Wiley & Sons, Inc.; 1990. pp. 93–130.
-
- Costerton J W, Cheng K J, Geesey G G, Ladd T I, Nickel J C, Dasgupta M, Marrie T J. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–464. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

