Role of methanogens and other bacteria in degradation of dimethyl sulfide and methanethiol in anoxic freshwater sediments
- PMID: 10224009
- PMCID: PMC91306
- DOI: 10.1128/AEM.65.5.2116-2121.1999
Role of methanogens and other bacteria in degradation of dimethyl sulfide and methanethiol in anoxic freshwater sediments
Abstract
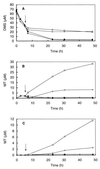
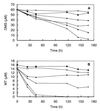
The roles of several trophic groups of organisms (methanogens and sulfate- and nitrate-reducing bacteria) in the microbial degradation of methanethiol (MT) and dimethyl sulfide (DMS) were studied in freshwater sediments. The incubation of DMS- and MT-amended slurries revealed that methanogens are the dominant DMS and MT utilizers in sulfate-poor freshwater systems. In sediment slurries, which were depleted of sulfate, 75 micromol of DMS was stoichiometrically converted into 112 micromol of methane. The addition of methanol or MT to DMS-degrading slurries at concentrations similar to that of DMS reduced DMS degradation rates. This indicates that the methanogens in freshwater sediments, which degrade DMS, are also consumers of methanol and MT. To verify whether a competition between sulfate-reducing and methanogenic bacteria for DMS or MT takes place in sulfate-rich freshwater systems, the effects of sulfate and inhibitors, like bromoethanesulfonic acid, molybdate, and tungstate, on the degradation of MT and DMS were studied. The results for these sulfate-rich and sulfate-amended slurry incubations clearly demonstrated that besides methanogens, sulfate-reducing bacteria take part in MT and DMS degradation in freshwater sediments, provided that sulfate is available. The possible involvement of an interspecies hydrogen transfer in these processes is discussed. In general, our study provides evidence for methanogenesis as a major sink for MT and DMS in freshwater sediments.
Figures



References
-
- Caron F, Kramer J R. Gas chromatographic determination of volatile sulfides at trace levels in natural freshwaters. Anal Chem. 1989;61:114–118.
-
- De Mello W Z, Hines M E. Application of static and dynamic enclosures for determining dimethylsulfide and carbonyl sulfide exchange in Sphagnum peatlands. J Geophys Res. 1994;99:14.601–14.607.
-
- De Mello, W. Z., and M. E. Hines. Emissions of dimethylsulfide from northern Sphagnum-dominated wetlands: controls on production and flux. Submitted for publication.
-
- De Zwart J M M, Nelisse P N, Kuenen J G. Isolation and characterization of Methylophaga sulfidovorans sp. nov.: an obligate methylotrophic aerobic, DMS oxidizing bacterium from a microbial mat. FEMS Microbiol Ecol. 1996;20:261–271.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

