Inhibition of T cell proliferation by macrophage tryptophan catabolism
- PMID: 10224276
- PMCID: PMC2193062
- DOI: 10.1084/jem.189.9.1363
Inhibition of T cell proliferation by macrophage tryptophan catabolism
Abstract
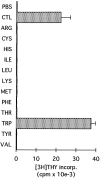
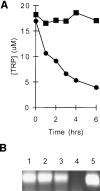
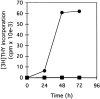
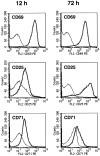
We have recently shown that expression of the enzyme indoleamine 2, 3-dioxygenase (IDO) during murine pregnancy is required to prevent rejection of the allogeneic fetus by maternal T cells. In addition to their role in pregnancy, IDO-expressing cells are widely distributed in primary and secondary lymphoid organs. Here we show that monocytes that have differentiated under the influence of macrophage colony-stimulating factor acquire the ability to suppress T cell proliferation in vitro via rapid and selective degradation of tryptophan by IDO. IDO was induced in macrophages by a synergistic combination of the T cell-derived signals IFN-gamma and CD40-ligand. Inhibition of IDO with the 1-methyl analogue of tryptophan prevented macrophage-mediated suppression. Purified T cells activated under tryptophan-deficient conditions were able to synthesize protein, enter the cell cycle, and progress normally through the initial stages of G1, including upregulation of IL-2 receptor and synthesis of IL-2. However, in the absence of tryptophan, cell cycle progression halted at a mid-G1 arrest point. Restoration of tryptophan to arrested cells was not sufficient to allow further cell cycle progression nor was costimulation via CD28. T cells could exit the arrested state only if a second round of T cell receptor signaling was provided in the presence of tryptophan. These data reveal a novel mechanism by which antigen-presenting cells can regulate T cell activation via tryptophan catabolism. We speculate that expression of IDO by certain antigen presenting cells in vivo allows them to suppress unwanted T cell responses.
Figures










References
-
- Fazekas de St. Groth B. The evolution of self-tolerance: a new cell arises to meet the challenge of self-reactivity. Immunol Today. 1998;19:448–454. - PubMed
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Munn DH, Armstrong E. Cytokine regulation of human monocyte differentiation in vitro: the tumor-cytotoxic phenotype induced by macrophage colony-stimulating factor is developmentally regulated by interferon γ. Cancer Res. 1993;53:2603–2613. - PubMed
-
- Munn DH, Pressey J, Beall AC, Hudes R, Alderson MR. Selective activation-induced apoptosis of peripheral T cells imposed by macrophages: a potential mechanism of antigen-specific peripheral lymphocyte deletion. J Immunol. 1996;156:523–532. - PubMed
-
- Shimizu T, Nomiyama S, Hirata F, Hayaishi O. Indoleamine 2,3-dioxygenase: purification and some properties. J Biol Chem. 1978;253:4700–4706. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

