Grf40, A novel Grb2 family member, is involved in T cell signaling through interaction with SLP-76 and LAT
- PMID: 10224278
- PMCID: PMC2193052
- DOI: 10.1084/jem.189.9.1383
Grf40, A novel Grb2 family member, is involved in T cell signaling through interaction with SLP-76 and LAT
Abstract
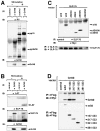
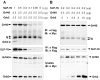
We molecularly cloned a new Grb2 family member, named Grf40, containing the common SH3-SH2-SH3 motif. Expression of Grf40 is predominant in hematopoietic cells, particularly T cells. Grf40 binds to the SH2 domain-containing leukocyte protein of 76 kD (SLP-76) via its SH3 domain more tightly than Grb2. Incidentally, Grf40 binds to linker for activation of T cells (LAT) possibly via its SH2 domain. Overexpression of wild-type Grf40 in Jurkat cells induced a significant increase of SLP-76-dependent interleukin (IL)-2 promoter and nuclear factor of activated T cell (NF-AT) activation upon T cell receptor (TCR) stimulation, whereas the COOH-terminal SH3-deleted Grf40 mutant lacked any recognizable increase in IL-2 promoter activity. Furthermore, the SH2-deleted Grf40 mutant led to a marked inhibition of these regulatory activities, the effect of which is apparently stronger than that of the SH2-deleted Grb2 mutant. Our data suggest that Grf40 is an adaptor molecule involved in TCR-mediated signaling through a more efficient interaction than Grb2 with SLP-76 and LAT.
Figures





References
-
- Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. - PubMed
-
- Cantrell D. T cell antigen receptor signal transduction pathways. Annu Rev Immunol. 1996;14:259–274. - PubMed
-
- Wange RL, Samelson LE. Complex complexes: signaling at the TCR. Immunity. 1996;5:197–205. - PubMed
-
- Koretzky GA. The role of Grb2-associated proteins in T-cell activation. Immunol Today. 1997;18:401–406. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

