Lymphocyte migration in lymphocyte function-associated antigen (LFA)-1-deficient mice
- PMID: 10224287
- PMCID: PMC2193056
- DOI: 10.1084/jem.189.9.1467
Lymphocyte migration in lymphocyte function-associated antigen (LFA)-1-deficient mice
Abstract
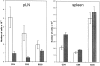
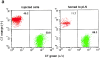
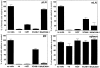
Using lymphocyte function-associated antigen (LFA)-1(-/-) mice, we have examined the role of LFA-1 and other integrins in the recirculation of lymphocytes. LFA-1 has a key role in migration to peripheral lymph nodes (pLNs), and influences migration into other LNs. Second, the alpha4 integrins, alpha4beta7 and alpha4beta1, have a hitherto unrecognized ability to compensate for the lack of LFA-1 in migration to pLNs. These findings are confirmed using normal mice and blocking LFA-1 and alpha4 monoclonal antibodies. Unexpectedly, vascular cell adhesion molecule (VCAM)-1, which is essential in inflammatory responses, serves as the ligand for the alpha4 integrins on pLN high endothelial venules. VCAM-1 also participates in trafficking into mesenteric LNs and Peyer's patch nodes where mucosal addressin cell adhesion molecule 1 (MAdCAM-1), the alpha4beta7-specific ligand, dominates. Both alpha4beta1, interacting with ligand VCAM-1, and also LFA-1 participate in substantial lymphocyte recirculation through bone marrow. These observations suggest that organ-specific adhesion receptor usage in mature lymphocyte recirculation is not as rigidly adhered to as previously considered, and that the same basic sets of adhesion receptors are used in both lymphocyte homing and inflammatory responses.
Figures




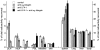
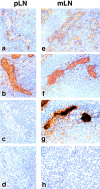
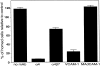
References
-
- Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. - PubMed
-
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. - PubMed
-
- Bargatze RF, Jutila MA, Butcher EC. Distinct roles of L-selectin and integrins α4β7 and LFA-1 in lymphocyte homing to Peyer's patch-HEV in situ: the multistep model confirmed and refined. Immunity. 1995;3:99–108. - PubMed
-
- Salmi M, Jalkanen S. How do lymphocytes know where to go: current concepts and enigmas of lymphocyte homing. Adv Immunol. 1997;64:139–218. - PubMed
-
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

