A new cell enzyme-linked immunosorbent assay demonstrates gamma interferon suppression by beta interferon in multiple sclerosis
- PMID: 10225846
- PMCID: PMC103733
- DOI: 10.1128/CDLI.6.3.415-419.1999
A new cell enzyme-linked immunosorbent assay demonstrates gamma interferon suppression by beta interferon in multiple sclerosis
Abstract
Multiple sclerosis (MS) is a demyelinating disorder of the central nervous system of unknown etiology. Immune mechanisms involving the proinflammatory cytokine gamma interferon (IFN-gamma) are believed to play an important role in the pathogenesis of MS. IFN-beta-1b has been introduced as a treatment for MS and was found to reduce the number and severity of clinical exacerbations. To examine the influence of IFN-beta-1b on myelin basic protein (MBP)-specific and phytohemagglutinin-induced IFN-gamma production, we developed a cell-released capturing enzyme-linked immunosorbent assay (CRC-ELISA), which rapidly measures spontaneous and antigen- or mitogen-induced cellular IFN-gamma production. CRC-ELISA documented a significant MBP-specific T-cell response in the blood of untreated MS patients, as assessed by IFN-gamma production. This response was suppressed in MS patients treated with IFN-beta-1b. The present work confirms in vivo the in vitro suppressive effects of IFN-beta-1b on IFN-gamma production in MS. Moreover, it provides a powerful new technique for detection of cytokines.
Figures
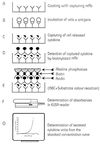
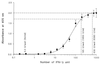
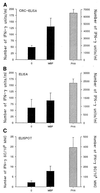
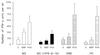
References
-
- Adams D O, Hamilton T A. Molecular transductional mechanisms by which IFN gamma and other signals regulate macrophage development. Immunol Rev. 1987;97:5–27. - PubMed
-
- Bakhiet, M., et al. Unpublished data.
-
- Banik N L. Pathogenesis of myelin breakdown in demyelinating diseases: role of proteolytic enzymes. Crit Rev Neurobiol. 1992;6:257–271. - PubMed
-
- Brod S A, Marshall G D, Henninger E M, Jr, Sriram S, Khan M, Wolinsky J S. IFN-β-1b treatment decreases tumor necrosis factor-α and increases interleukin-6 production in multiple sclerosis. Neurology. 1996;46:1633–1638. - PubMed
-
- Cruz M, Olsson T, Ernerudh J, Höjeberg B, Link H. Immunoblot detection of oligoclonal anti-myelin basic protein IgG antibodies in cerebrospinal fluid in multiple sclerosis. Neurology. 1987;37:1515–1519. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

