Role of polymorphonuclear neutrophils in a murine model of Chlamydia psittaci-induced abortion
- PMID: 10225862
- PMCID: PMC115945
- DOI: 10.1128/IAI.67.5.2110-2116.1999
Role of polymorphonuclear neutrophils in a murine model of Chlamydia psittaci-induced abortion
Abstract
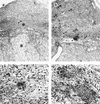
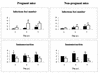
To assess the role of polymorphonuclear neutrophils (PMNs) in Chlamydia psittaci infection in a pregnant mouse model, pregnant and nonpregnant Swiss OF1 mice were depleted of PMNs by treatment with the RB6-8C5 monoclonal antibody before intraperitoneal infection with C. psittaci serotype 1. Nondepleted mice served as infection controls. Depleted mice aborted earlier and had a much higher mortality rate than nondepleted mice. Bacteriological analysis showed that the number of chlamydiae isolated from the spleens of depleted mice at 5 and 7 days postinfection was 100 times greater than that isolated from nondepleted mice. Histopathological analysis of the placentas of depleted mice showed widespread necrosis of the uteroplacental units, with weak immunoreaction to chlamydial antigen, while the placentas of nondepleted mice showed substantial neutrophil infiltration but no large areas of necrosis, with moderate to strong immunoreaction to chlamydial antigen. The livers of depleted mice showed numerous chlamydial inclusions in the hepatocytes, delayed microgranuloma formation, and in the pregnant animals extensive coagulative periportal necrosis. The livers of nondepleted mice displayed multiple small foci of PMNs and mononuclear cells with microgranuloma formation. Among this group of mice, the pregnant animals always had more hepatic damage than nonpregnant animals. Our results suggest that PMNs play an essential role in the response to C. psittaci primary infection, preventing the uncontrolled multiplication of chlamydiae in the liver and spleen.
Figures

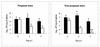

References
-
- Buxton D, Barlow R M, Finlayson J, Anderson I E, Mackellar A. Observations on the pathogenesis of Chlamydia psittaci infection of pregnant sheep. J Comp Pathol. 1990;102:221–237. - PubMed
-
- Cassatella M A. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

