Cytoskeletal effects induced by pet, the serine protease enterotoxin of enteroaggregative Escherichia coli
- PMID: 10225873
- PMCID: PMC115956
- DOI: 10.1128/IAI.67.5.2184-2192.1999
Cytoskeletal effects induced by pet, the serine protease enterotoxin of enteroaggregative Escherichia coli
Abstract
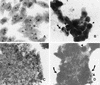
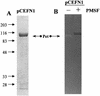
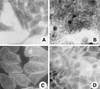
We have previously described enteroaggregative Escherichia coli (EAEC) strains that induce cytotoxic effects on T84 cells, ligated rat ileal loops, and human intestine in culture. Such strains secrete a 104-kDa protein termed Pet (for plasmid-encoded toxin). We have also shown previously that the Pet toxin induces rises in short-circuit current and decreases the electrical resistance in rat jejunum mounted in an Ussing chamber. The nucleotide sequence of the pet gene revealed that Pet is a member of the autotransporter class of secreted proteins. Here we show that a concentrated supernatant of E. coli HB101 harboring the minimal pet clone pCEFN1 induces temperature-, time- and dose-dependent cytopathic effects on HEp-2 cells and HT29 C1 cells in culture. The effects were characterized by release of the cellular focal contacts from the glass substratum, followed by complete rounding of the cells and detachment from the glass. Staining of the Pet-treated cells with Live/Dead viability stain revealed that >90% of rounded cells were viable. Pet-intoxicated HEp-2 and HT29 cells stained with fluorescein-labeled phalloidin revealed contraction of the cytoskeleton and loss of actin stress fibers. However, the effects of Pet were not inhibited by cytoskeleton-altering drugs, including colchicine, taxol, cytochalasin D, and phallicidin. The Pet protein induced proteolysis in zymogram gels, and preincubation with the serine protease inhibitor phenylmethylsulfonyl fluoride resulted in complete abrogation of Pet cytopathic effects. We introduced a mutation in a predicted catalytic serine residue and found that the mutant (Pet S260I) was deficient in protease activity and did not produce cytopathic effects, cytoskeletal damage, or enterotoxic effects in Ussing chambers. These data suggest that Pet is a cytoskeleton-altering toxin and that its protease activity is involved in each of the observed phenotypes.
Figures



References
-
- Benjelloun-Touimi Z, Tahar M S, Montecucco C, Sansonetti P J, Parsot C. SepA, the 110 kDa protein secreted by Shigella flexneri: two-domain structure and proteolytic activity. Microbiology. 1995;144:1815–1822. - PubMed
-
- Bhan M K, Raj P, Levine M M, Kaper J B, Bhandari N, Srivastava R, Kumar R, Sazawal S. Enteroaggregative Escherichia coli associated with persistent diarrhea in a cohort of rural children in India. J Infect Dis. 1989;159:1061–1064. - PubMed
-
- Brunder W, Schmidt H, Karch H. EspP, a novel extracellular serine protease of enterohaemorrhagic Escherichia coli O157:H7 cleaves human coagulation factor V. Mol Microbiol. 1997;24:767–78. - PubMed
-
- Cravioto A, Tello A, Navarro A, Ruiz J, Villafan H, Uribe F, Eslava C. Association of Escherichia coli HEp-2 adherence patterns with type and duration of diarrhoea. Lancet. 1991;337:262–264. - PubMed
-
- de Bernard M, Papini E, de Filippis V, Gottardi E, Telford J, Manetti R, Fontana A, Rappuoli R, Montecucco C. Low pH activates the vacuolating toxin of Helicobacter pylori, which becomes acid and pepsin resistant. J Biol Chem. 1995;270:23937–23940. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

