Cellular immune responses to Neisseria meningitidis in children
- PMID: 10225908
- PMCID: PMC115991
- DOI: 10.1128/IAI.67.5.2452-2463.1999
Cellular immune responses to Neisseria meningitidis in children
Abstract
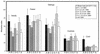
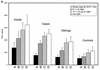
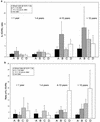
There is an urgent need for effective vaccines against serogroup B Neisseria meningitidis. Current experimental vaccines based on the outer membrane proteins (OMPs) of this organism provide a measure of protection in older children but have been ineffective in infants. We postulated that the inability of OMP vaccines to protect infants might be due to age-dependent defects in cellular immunity. We measured proliferation and in vitro production of gamma interferon (IFN-gamma), tumor necrosis factor alpha, and interleukin-10 (IL-10) in response to meningococcal antigens by peripheral blood mononuclear cells (PBMCs) from children convalescing from meningococcal disease and from controls. After meningococcal infection, the balance of cytokine production by PBMCs from the youngest children was skewed towards a TH1 response (low IL-10/IFN-gamma ratio), while older children produced more TH2 cytokine (higher IL-10/IFN-gamma ratio). There was a trend to higher proliferative responses by PBMCs from older children. These responses were not influenced by the presence or subtype of class 1 (PorA) OMP or by the presence of class 2/3 (PorB) or class 4 OMP. Even young infants might be expected to develop adequate cellular immune responses to serogroup B N. meningitidis vaccines if a vaccine preparation can be formulated to mimic the immune stimulus of invasive disease, which may include stimulation of TH2 cytokine production.
Figures




Similar articles
-
Assessment of the direct effectiveness of BC meningococcal vaccine in Rio de Janeiro, Brazil: a case-control study.Int J Epidemiol. 1995 Oct;24(5):1050-7. doi: 10.1093/ije/24.5.1050. Int J Epidemiol. 1995. PMID: 8557439
-
Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile.JAMA. 1999 Apr 28;281(16):1520-7. doi: 10.1001/jama.281.16.1520. JAMA. 1999. PMID: 10227322 Clinical Trial.
-
Humoral immune responses to Neisseria meningitidis in children.Infect Immun. 1999 May;67(5):2441-51. doi: 10.1128/IAI.67.5.2441-2451.1999. Infect Immun. 1999. PMID: 10225907 Free PMC article.
-
Challenges for development of meningococcal vaccines in infants and children.Expert Rev Vaccines. 2011 Mar;10(3):335-43. doi: 10.1586/erv.11.3. Expert Rev Vaccines. 2011. PMID: 21434801 Review.
-
Serogroup B meningococcal vaccines-an unfinished story.Lancet Infect Dis. 2010 Feb;10(2):112-24. doi: 10.1016/S1473-3099(09)70324-X. Lancet Infect Dis. 2010. PMID: 20113980 Review.
Cited by
-
Age and disease related changes in intestinal bacterial populations assessed by cell culture, 16S rRNA abundance, and community cellular fatty acid profiles.Gut. 2001 Feb;48(2):198-205. doi: 10.1136/gut.48.2.198. Gut. 2001. PMID: 11156640 Free PMC article.
-
Characterization of humoral and cellular immune responses elicited by meningococcal carriage.Infect Immun. 2002 Mar;70(3):1301-9. doi: 10.1128/IAI.70.3.1301-1309.2002. Infect Immun. 2002. PMID: 11854214 Free PMC article.
-
Cellular Immune Responses in Humans Induced by Two Serogroup B Meningococcal Outer Membrane Vesicle Vaccines Given Separately and in Combination.Clin Vaccine Immunol. 2016 Apr 4;23(4):353-62. doi: 10.1128/CVI.00666-15. Print 2016 Apr. Clin Vaccine Immunol. 2016. PMID: 26865595 Free PMC article.
-
Characterisation of the Immunomodulatory Effects of Meningococcal Opa Proteins on Human Peripheral Blood Mononuclear Cells and CD4+ T Cells.PLoS One. 2016 Apr 25;11(4):e0154153. doi: 10.1371/journal.pone.0154153. eCollection 2016. PLoS One. 2016. PMID: 27111850 Free PMC article.
-
How the Knowledge of Interactions between Meningococcus and the Human Immune System Has Been Used to Prepare Effective Neisseria meningitidis Vaccines.J Immunol Res. 2015;2015:189153. doi: 10.1155/2015/189153. Epub 2015 Aug 17. J Immunol Res. 2015. PMID: 26351643 Free PMC article. Review.
References
-
- Abbas A K, Murphy K M, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. - PubMed
-
- Abdillahi H, Poolman J T. Neisseria meningitidis group B serosubtyping using monoclonal antibodies in whole-cell ELISA. Microb Pathog. 1988;4:27–32. - PubMed
-
- Abdillahi H, Poolman J T. Whole-cell ELISA for typing Neisseria meningitidis with monoclonal antibodies. FEMS Microbiol Lett. 1987;48:367–371. - PubMed
-
- Ala’Aldeen D A. Transferrin receptors of Neisseria meningitidis: promising candidates for a broadly cross-protective vaccine. J Med Microbiol. 1996;44:237–243. - PubMed
-
- Anonymous. 1995 communicable disease statistics, vol. 22. Office of National Statistics. London, United Kingdom: HMSO Books; 1997.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

