Trafficking of an acylated cytosolic protein: newly synthesized p56(lck) travels to the plasma membrane via the exocytic pathway
- PMID: 10225948
- PMCID: PMC2185081
- DOI: 10.1083/jcb.145.3.457
Trafficking of an acylated cytosolic protein: newly synthesized p56(lck) travels to the plasma membrane via the exocytic pathway
Abstract
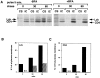
The Src-related tyrosine kinase p56(lck) (Lck) is primarily expressed in T lymphocytes where it localizes to the cytosolic side of the plasma membrane and associates with the T cell coreceptors CD4 and CD8. As a model for acylated proteins, we studied how this localization of Lck is achieved. We followed newly synthesized Lck by pulse-chase analysis and found that membrane association of Lck starts soon after synthesis, but is not complete until at least 30-45 min later. Membrane-binding kinetics are similar in CD4/CD8-positive and CD4/CD8-negative cells. In CD4-positive T cells, the interaction with CD4 rapidly follows membrane association of Lck. Studying the route via which Lck travels from its site of synthesis to the plasma membrane, we found that: CD4 associates with Lck within 10 min of synthesis, long before CD4 has reached the plasma membrane; Lck associates with intracellular CD4 early after synthesis and with cell surface CD4 at later times; and transport of CD4-bound Lck to the plasma membrane is inhibited by Brefeldin A. These data indicate that the initial association of newly synthesized Lck with CD4, and therefore with membranes, occurs on intracellular membranes of the exocytic pathway. From this location Lck is transported to the plasma membrane.
Figures
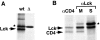





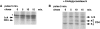

References
-
- Alland L, Peseckis SM, Atherton RE, Berthiaume L, Resh MD. Dual myristylation and palmitylation of Src family member p59fyn affects subcellular localization. J Biol Chem. 1994;24:16701–16705. - PubMed
-
- Berthiaume L, Resh MD. Biochemical characterization of a palmitoyl acyltransferase activity that palmitoylates myristoylated proteins. J Biol Chem. 1995;270:22399–22405. - PubMed
-
- Bhatnagar RS, Gordon JI. Understanding covalent modifications of proteins by lipids: where cell biology and biophysics mingle. Trends Cell Biol. 1997;7:14–21. - PubMed
-
- Bonatti S, Migliaccio G, Simons K. Palmitylation of viral membrane glycoproteins takes place after exit from the endoplasmic reticulum. J Biol Chem. 1989;264:12590–12595. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

