Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vimentin
- PMID: 10225952
- PMCID: PMC2185074
- DOI: 10.1083/jcb.145.3.503
Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vimentin
Abstract
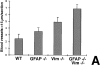
In response to injury of the central nervous system, astrocytes become reactive and express high levels of the intermediate filament (IF) proteins glial fibrillary acidic protein (GFAP), vimentin, and nestin. We have shown that astrocytes in mice deficient for both GFAP and vimentin (GFAP-/-vim-/-) cannot form IFs even when nestin is expressed and are thus devoid of IFs in their reactive state. Here, we have studied the reaction to injury in the central nervous system in GFAP-/-, vimentin-/-, or GFAP-/-vim-/- mice. Glial scar formation appeared normal after spinal cord or brain lesions in GFAP-/- or vimentin-/- mice, but was impaired in GFAP-/-vim-/- mice that developed less dense scars frequently accompanied by bleeding. These results show that GFAP and vimentin are required for proper glial scar formation in the injured central nervous system and that some degree of functional overlap exists between these IF proteins.
Figures










References
-
- Bignami A, Raju T, Dahl D. Localization of vimentin, the nonspecific intermediate filament protein, in embryonal glia and in early differentiating neurons. In vivo and in vitro immunofluorescence study of the rat embryo with vimentin and neurofilament antisera. Dev Biol. 1982;91:286–295. - PubMed
-
- Bovolenta P, Liem RK, Mason CA. Development of cerebellar astroglia: transition in form and cytoskeletal content. Dev Biol. 1984;102:248–259. - PubMed
-
- Clarke SR, Shetty AK, Bradley JL, Turner DA. Reactive astrocytes express the embryonic intermediate neurofilament nestin. Neuroreport. 1994;5:1885–1888. - PubMed
-
- Colucci-Guyon E, Portier MM, Dunia I, Paulin D, Pournin S, Babinet C. Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell. 1994;79:679–694. - PubMed
-
- Dahlstrand J, Collins VP, Lendahl U. Expression of the class VI intermediate filament nestin in human central nervous system tumors. Cancer Res. 1992;52:5334–5341. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

