p120(ctn) acts as an inhibitory regulator of cadherin function in colon carcinoma cells
- PMID: 10225956
- PMCID: PMC2185070
- DOI: 10.1083/jcb.145.3.551
p120(ctn) acts as an inhibitory regulator of cadherin function in colon carcinoma cells
Abstract
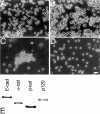
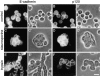
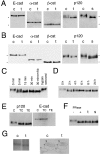
p120(ctn) binds to the cytoplasmic domain of cadherins but its role is poorly understood. Colo 205 cells grow as dispersed cells despite their normal expression of E-cadherin and catenins. However, in these cells we can induce typical E-cadherin-dependent aggregation by treatment with staurosporine or trypsin. These treatments concomitantly induce an electrophoretic mobility shift of p120(ctn) to a faster position. To investigate whether p120(ctn) plays a role in this cadherin reactivation process, we transfected Colo 205 cells with a series of p120(ctn) deletion constructs. Notably, expression of NH2-terminally deleted p120(ctn) induced aggregation. Similar effects were observed when these constructs were introduced into HT-29 cells. When a mutant N-cadherin lacking the p120(ctn)-binding site was introduced into Colo 205 cells, this molecule also induced cell aggregation, indicating that cadherins can function normally if they do not bind to p120(ctn). These findings suggest that in Colo 205 cells, a signaling mechanism exists to modify a biochemical state of p120(ctn) and the modified p120(ctn) blocks the cadherin system. The NH2 terminus-deleted p120(ctn) appears to compete with the endogenous p120(ctn) to abolish the adhesion-blocking action.
Figures





References
-
- Baeckstrom D, Hansson GC, Nilsson O, Johansson C, Gendler SJ, Lindholm L. Purification and characterization of a membrane-bound and a secreted mucin-type glycoprotein carrying the carcinoma-associated sialyl-Lea epitope on distinct core proteins. J Biol Chem. 1991;266:21537–21547. - PubMed
-
- Barth AIM, Näthke IS, Nelson WJ. Cadherins, catenins and APC protein: interplay between cytoskeletal complexes and signaling pathways. Curr Opin Cell Biol. 1997;9:683–690. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

