Mouse ten-m/Odz is a new family of dimeric type II transmembrane proteins expressed in many tissues
- PMID: 10225957
- PMCID: PMC2185078
- DOI: 10.1083/jcb.145.3.563
Mouse ten-m/Odz is a new family of dimeric type II transmembrane proteins expressed in many tissues
Abstract
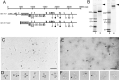
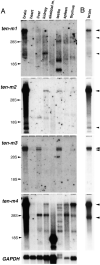
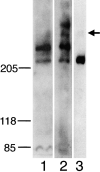
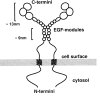
The Drosophila gene ten-m/odz is the only pair rule gene identified to date which is not a transcription factor. In an attempt to analyze the structure and the function of ten-m/odz in mouse, we isolated four murine ten-m cDNAs which code for proteins of 2,700-2, 800 amino acids. All four proteins (Ten-m1-4) lack signal peptides at the NH2 terminus, but contain a short hydrophobic domain characteristic of transmembrane proteins, 300-400 amino acids after the NH2 terminus. About 200 amino acids COOH-terminal to this hydrophobic region are eight consecutive EGF-like domains. Cell transfection, biochemical, and electronmicroscopic studies suggest that Ten-m1 is a dimeric type II transmembrane protein. Expression of fusion proteins composed of the NH2-terminal and hydrophobic domain of ten-m1 attached to the alkaline phosphatase reporter gene resulted in membrane-associated staining of the alkaline phosphatase. Electronmicroscopic and electrophoretic analysis of a secreted form of the extracellular domain of Ten-m1 showed that Ten-m1 is a disulfide-linked dimer and that the dimerization is mediated by EGF-like modules 2 and 5 which contain an odd number of cysteines. Northern blot and immunohistochemical analyses revealed widespread expression of mouse ten-m genes, with most prominent expression in brain. All four ten-m genes can be expressed in variously spliced mRNA isoforms. The extracellular domain of Ten-m1 fused to an alkaline phosphatase reporter bound to specific regions in many tissues which were partially overlapping with the Ten-m1 immunostaining. Far Western assays and electronmicroscopy demonstrated that Ten-m1 can bind to itself.
Figures



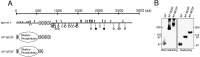


References
-
- Altschul SF, Boguski MS, Gish W, Wootton JC. Issues in searching molecular sequence databases. Nat Genet. 1994;6:119–129. - PubMed
-
- Barr PJ. Mammalian subtilisins: the long-sought dibasic processing endoproteases. Cell. 1991;66:1–3. - PubMed
-
- Baumgartner S, Chiquet-Ehrismann R. Tena, a Drosophilagene related to tenascin, shows selective transcript localization. Mech Dev. 1993;40:165–176. - PubMed
-
- Binari R, Perrimon N. Stripe-specific regulation of pair-rule genes by hopscotch, a putative Jak family tyrosine kinase in Drosophila. . Genes Dev. 1994;8:300–312. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

