Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization
- PMID: 10225965
- PMCID: PMC408362
- DOI: 10.1172/JCI6889
Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization
Figures
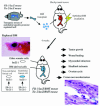
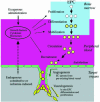

References
-
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. - PubMed
-
- Ware JA, Simons M. Angiogenesis in ischemic heart disease. Nat Med. 1997;3:158–164. - PubMed
-
- Mack CA, et al. Biologic bypass with the use of adenovirus-mediated gene transfer of the complementary deoxyribonucleic acid for vascular endothelial growth factor 121 improves myocardial perfusion and function in the ischemic porcine heart. J Thorac Cardiovasc Surg. 1998;115:168–176. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

