G-protein coupled receptor kinases as modulators of G-protein signalling
- PMID: 10226145
- PMCID: PMC2269335
- DOI: 10.1111/j.1469-7793.1999.0005z.x
G-protein coupled receptor kinases as modulators of G-protein signalling
Abstract
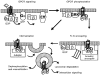
G-protein coupled receptors (GPCRs) comprise one of the largest classes of signalling molecules. A wide diversity of activating ligands induce the active conformation of GPCRs and lead to signalling via heterotrimeric G-proteins and downstream effectors. In addition, a complex series of reactions participate in the 'turn-off' of GPCRs in both physiological and pharmacological settings. Some key players in the inactivation or 'desensitization' of GPCRs have been identified, whereas others remain the target of ongoing studies. G-protein coupled receptor kinases (GRKs) specifically phosphorylate activated GPCRs and initiate homologous desensitization. Uncoupling proteins, such as members of the arrestin family, bind to the phosphorylated and activated GPCRs and cause desensitization by precluding further interactions of the GPCRs and G-proteins. Adaptor proteins, including arrestins, and endocytic machinery participate in the internalization of GPCRs away from their normal signalling milieu. In this review we discuss the roles of these regulatory molecules as modulators of GPCR signalling.
Figures
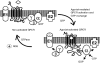

References
-
- Akhter SA, Skaer CA, Kypson AP, McDonald PH, Peppel KC, Glower DD, Lefkowitz RJ, Koch WJ. Restoration of β-adrenergic signaling in failing cardiac ventricular myocytes via adenoviral-mediated gene transfer. Proceedings of the National Academy of Sciences of the USA. 1997;94:12100–12105. - PMC - PubMed
-
- Appleyard SM, Patterson TA, Jin W, Chavkin C. Agonist-induced phosphorylation of the κ-opioid receptor. Journal of Neurochemistry. 1997;69:2405–2412. - PubMed
-
- Aragay AM, Mellado M, Frade JM, Martin AM, Jimenez-Sainz MC, Martinez AC, Mayor F., Jr Monocyte chemoattractant protein-1-induced CCR2B receptor desensitization mediated by the G protein-coupled receptor kinase 2. Proceedings of the National Academy of Sciences of the USA. 1998a;95:2985–2990. - PMC - PubMed
-
- Aragay AM, Ruiz-Gomez A, Penela P, Sarnago S, Elorza A, Jimenez-Sainz MC, Mayor F., Jr G protein-coupled receptor kinase 2 (GRK2): mechanisms of regulation and physiological functions. FEBS Letters. 1998b;430:37–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

