Modulation of neuronal and recombinant GABAA receptors by redox reagents
- PMID: 10226147
- PMCID: PMC2269321
- DOI: 10.1111/j.1469-7793.1999.0035z.x
Modulation of neuronal and recombinant GABAA receptors by redox reagents
Abstract
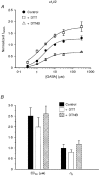
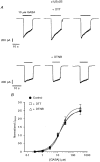
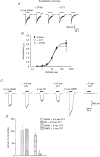
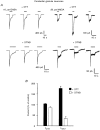
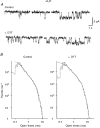
1. The functional role played by the postulated disulphide bridge in gamma-aminobutyric acid type A (GABAA) receptors and its susceptibility to oxidation and reduction were studied using recombinant (murine receptor subunits expressed in human embryonic kidney cells) and rat neuronal GABAA receptors in conjunction with whole-cell and single channel patch-clamp techniques. 2. The reducing agent dithiothreitol (DTT) reversibly potentiated GABA-activated responses (IGABA) of alpha1beta1 or alpha1beta2 receptors while the oxidizing reagent 5, 5'-dithio-bis-(2-nitrobenzoic acid) (DTNB) caused inhibition. Redox modulation of IGABA was independent of GABA concentration, membrane potential and the receptor agonist and did not affect the GABA EC50 or Hill coefficient. The endogenous antioxidant reduced glutathione (GSH) also potentiated IGABA in alpha1beta2 receptors, while both the oxidized form of DTT and glutathione (GSSG) caused small inhibitory effects. 3. Recombinant receptors composed of alpha1beta1gamma2S or alpha1beta2gamma2S were considerably less sensitive to DTT and DTNB. 4. For neuronal GABAA receptors, IGABA was enhanced by flurazepam and relatively unaffected by redox reagents. However, in cultured sympathetic neurones, nicotinic acetylcholine-activated responses were inhibited by DTT whilst in cerebellar granule neurones, NMDA-activated currents were potentiated by DTT and inhibited by DTNB. 5. Single GABA-activated ion channel currents exhibited a conductance of 16 pS for alpha1beta1 constructs. DTT did not affect the conductance or individual open time constants determined from dwell time histograms, but increased the mean open time by affecting the channel open probability without increasing the number of cell surface receptors. 6. A kinetic model of the effects of DTT and DTNB suggested that the receptor existed in equilibrium between oxidized and reduced forms. DTT increased the rate of entry into reduced receptor forms and also into desensitized states. DTNB reversed these kinetic effects. 7. Our results indicate that GABAA receptors formed by alpha and beta subunits are susceptible to regulation by redox agents. Inclusion of the gamma2 subunit in the receptor, or recording from some neuronal GABAA receptors, resulted in reduced sensitivity to DTT and DTNB. Given the suggested existence of alphabeta subunit complexes in some areas of the central nervous system together with the generation and release of endogenous redox compounds, native GABAA receptors may be subject to regulation by redox mechanisms.
Figures




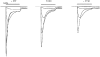
References
-
- Aizenman E, Lipton SA, Loring RH. Selective modulation of NMDA responses by reduction and oxidation. Neuron. 1989;2:1257–1263. 10.1016/0896-6273(89)90310-3. - DOI - PubMed
-
- Amin J, Dickerson IM, Weiss DS. The agonist binding site of the γ-aminobutyric acid type A channel is not formed by the extracellular cysteine loop. Molecular Pharmacology. 1994;45:317–323. - PubMed
-
- Barnard EA, Darlison MG, Seeburg P. Molecular biology of the GABAA receptor: the receptor/channel superfamily. Trends in Neurosciences. 1987;10:502–509.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

