A non-capacitative pathway activated by arachidonic acid is the major Ca2+ entry mechanism in rat A7r5 smooth muscle cells stimulated with low concentrations of vasopressin
- PMID: 10226154
- PMCID: PMC2269333
- DOI: 10.1111/j.1469-7793.1999.0121z.x
A non-capacitative pathway activated by arachidonic acid is the major Ca2+ entry mechanism in rat A7r5 smooth muscle cells stimulated with low concentrations of vasopressin
Abstract
1. Depletion of the Ca2+ stores of A7r5 cells stimulated Ca2+, though not Sr2+, entry. Vasopressin (AVP) or platelet-derived growth factor (PDGF) stimulated Sr2+ entry. The cells therefore express a capacitative pathway activated by empty stores and a non-capacitative pathway stimulated by receptors; only the former is permeable to Mn2+ and only the latter to Sr2+. 2. Neither empty stores nor inositol 1,4,5-trisphosphate (InsP3) binding to its receptors are required for activation of the non-capacitative pathway, because microinjection of cells with heparin prevented PDGF-evoked Ca2+ mobilization but not Sr2+ entry. 3. Low concentrations of Gd3+ irreversibly blocked capacitative Ca2+ entry without affecting AVP-evoked Sr2+ entry. After inhibition of the capacitative pathway with Gd3+, AVP evoked a substantial increase in cytosolic [Ca2+], confirming that the non-capacitative pathway can evoke a significant increase in cytosolic [Ca2+]. 4. Arachidonic acid mimicked the effect of AVP on Sr2+ entry without stimulating Mn2+ entry; the Sr2+ entry was inhibited by 100 microM Gd3+, but not by 1 microM Gd3+ which completely inhibited capacitative Ca2+ entry. The effects of arachidonic acid did not require its metabolism. 5. AVP-evoked Sr2+ entry was unaffected by isotetrandrine, an inhibitor of G protein-coupled phospholipase A2. U73122, an inhibitor of phosphoinositidase C, inhibited AVP-evoked formation of inositol phosphates and Sr2+ entry. The effects of phorbol esters and Ro31-8220 (a protein kinase C inhibitor) established that protein kinase C did not mediate the effects of AVP on the non-capacitative pathway. An inhibitor of diacylglycerol lipase, RHC-80267, inhibited AVP-evoked Sr2+ entry without affecting capacitative Ca2+ entry or release of Ca2+ stores. 6. Selective inhibition of capacitative Ca2+ entry with Gd3+ revealed that the non-capacitative pathway is the major route for the Ca2+ entry evoked by low AVP concentrations. 7. We conclude that in A7r5 cells, the Ca2+ entry evoked by low concentrations of AVP is mediated largely by a non-capacitative pathway directly regulated by arachidonic acid produced by the sequential activities of phosphoinositidase C and diacylglycerol lipase.
Figures
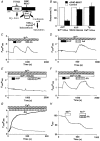
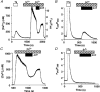

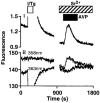
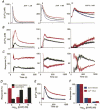
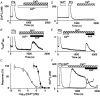
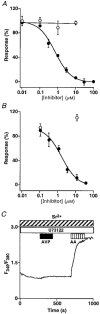
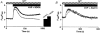
Comment in
-
Activator of calcium influx proves a slippery customer.J Physiol. 1999 May 15;517 ( Pt 1)(Pt 1):2. doi: 10.1111/j.1469-7793.1999.0002z.x. J Physiol. 1999. PMID: 10226143 Free PMC article. No abstract available.
References
-
- Akiba S, Kato E, Sato T, Fujii T. Biscolaurine alkaloids inhibit receptor-mediated phospholipase A2 activation probably through uncoupling of a GTP-binding protein from the enzyme in rat peritoneal mast cells. Biochemical Pharmacology. 1992;44:45–50. 10.1016/0006-2952(92)90036-I. - DOI - PubMed
-
- Alonso-Torre SR, Garciá-Sancho J. Arachidonic acid inhibits capacitative calcium entry in rat thymocytes and human neutrophils. Biochimica et Biophysica Acta. 1997;1328:207–213. - PubMed
-
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. - PubMed
-
- Bleasdale JE, Thakur NR, Gremban RS, Bundy GL, Fitzpatrick FA, Smith RJ, Bunting S. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. Journal of Pharmacology and Experimental Therapeutics. 1990;255:756–768. - PubMed
-
- Byron KL, Taylor CW. Spontaneous Ca2+ spiking in a vascular smooth muscle cell line is independent of the release of intracellular Ca2+ stores. Journal of Biological Chemistry. 1993;268:6945–6952. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

