Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation
- PMID: 10229805
- PMCID: PMC2228333
Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation
Abstract
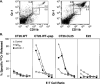
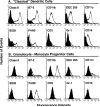
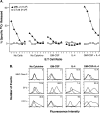
Tumor cells gene-modified to produce GM-CSF potently stimulate antitumor immune responses, in part, by causing the growth and differentiation of dendritic cells (DC). However, GM-CSF-modified tumor cells must be gamma-irradiated or they will grow progressively, killing the host. We observed that 23 of 75 (31%) human tumor lines and two commonly used mouse tumor lines spontaneously produced GM-CSF. In mice, chronic GM-CSF production by tumors suppressed Ag-specific CD8+ T cell responses. Interestingly, an inhibitory population of adherent CD11b(Mac-1)/Gr-1 double-positive cells caused the observed impairment of CD8+ T cell function upon direct cell-to-cell contact. The inhibitory cells were positive for some markers associated with Ag presenting cells, like F4/80, but were negative for markers associated with fully mature DC like DEC205, B7. 2, and MHC class II. We have previously reported that a similar or identical population of inhibitory "immature" APC was elicited after immunization with powerful recombinant immunogens. We show here that these inhibitory cells can be elicited by the administration of recombinant GM-CSF alone, and, furthermore, that they can be differentiated ex vivo into "mature" APC by the addition of IL-4 and GM-CSF. Thus, tumors may be able to escape from immune detection by producing "unopposed" GM-CSF, thereby disrupting the balance of cytokines needed for the maturation of fully functional DC. Further, CD11b/Gr-1 double-positive cells may function as "inhibitory" APC under the influence of GM-CSF alone.
Figures


References
-
- Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, Tsung K, Carroll MW, Liu C, Moss B, Rosenberg SA, Restifo NP. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J. Exp. Med. 1998;188:277. - PMC - PubMed
-
- Overwijk WW, Lee DS, Surman DR, Irvine KR, Touloukian CE, Chan C-C, Carroll MW, Moss B, Rosenberg SA, Restifo NP. Vaccination with a recombinant vaccinia virus encoding a “self” antigen induces autoimmune vitiligo and tumor destruction in mice: Requirement for CD4+ T lymphocytes. Proc. Natl. Acad. Sci. USA. 1999 In press. - PMC - PubMed
-
- Pan ZK, Ikonomidis G, Lazenby A, Pardoll D, Paterson Y. A recombinant Listeria monocytogenes vaccine expressing a model tumour antigen protects mice against lethal tumour cell challenge and causes regression of established tumours. Nat. Med. 1995;1:471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
