Association of myosin I alpha with endosomes and lysosomes in mammalian cells
- PMID: 10233157
- PMCID: PMC25307
- DOI: 10.1091/mbc.10.5.1477
Association of myosin I alpha with endosomes and lysosomes in mammalian cells
Abstract
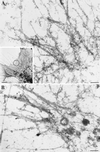
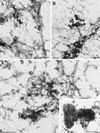
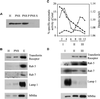
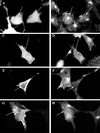
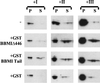
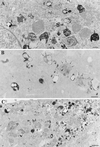
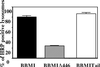
Myosin Is, which constitute a ubiquitous monomeric subclass of myosins with actin-based motor properties, are associated with plasma membrane and intracellular vesicles. Myosin Is have been proposed as key players for membrane trafficking in endocytosis or exocytosis. In the present paper we provide biochemical and immunoelectron microscopic evidence indicating that a pool of myosin I alpha (MMIalpha) is associated with endosomes and lysosomes. We show that the overproduction of MMIalpha or the production of nonfunctional truncated MMIalpha affects the distribution of the endocytic compartments. We also show that truncated brush border myosin I proteins, myosin Is that share 78% homology with MMIalpha, promote the dissociation of MMIalpha from vesicular membranes derived from endocytic compartments. The analysis at the ultrastructural level of cells producing these brush border myosin I truncated proteins shows that the delivery of the fluid phase markers from endosomes to lysosomes is impaired. MMIalpha might therefore be involved in membrane trafficking occurring between endosomes and lysosomes.
Figures

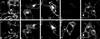


References
-
- Bailly E, Favel J, Mahouy G, Jaureguyberry G. Plasmodium falsiparum: isolation and characterization of a 55 kDa protease with cathepsin like activity from Plasmodium falsiparum. Exp Parasitol. 1991;72:278–284. - PubMed
-
- Baker JP, Titus MA. Myosins: matching functions with motors. Curr Opin Cell Biol. 1998;10:80–86. - PubMed
-
- Bakker AC, Webster P, Jacob WA, Andrews NW. Homotypic fusion between aggregated lysosomes triggered by elevated (Ca2+) in fibroblasts. J Cell Sci. 1997;110:2227–2238. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

