Radixin is involved in lamellipodial stability during nerve growth cone motility
- PMID: 10233159
- PMCID: PMC25322
- DOI: 10.1091/mbc.10.5.1511
Radixin is involved in lamellipodial stability during nerve growth cone motility
Abstract
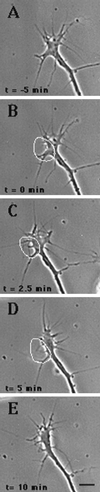
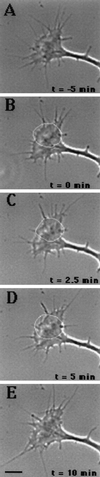
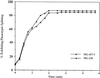
Immunocytochemistry and in vitro studies have suggested that the ERM (ezrin-radixin-moesin) protein, radixin, may have a role in nerve growth cone motility. We tested the in situ role of radixin in chick dorsal root ganglion growth cones by observing the effects of its localized and acute inactivation. Microscale chromophore-assisted laser inactivation (micro-CALI) of radixin in growth cones causes a 30% reduction of lamellipodial area within the irradiated region whereas all control treatments did not affect lamellipodia. Micro-CALI of radixin targeted to the middle of the leading edge often split growth cones to form two smaller growth cones during continued forward movement (>80%). These findings suggest a critical role for radixin in growth cone lamellipodia that is similar to ezrin function in pseudopodia of transformed fibroblasts. They are consistent with radixin linking actin filaments to each other or to the membrane during motility.
Figures

References
-
- Arpin M, Algrain M, Louvard D. Membrane-actin microfilament connection: an increasing diversity of players related to band 4.1. Curr Opin Cell Biol. 1994;6:136–141. - PubMed
-
- Beerman AE, Jay DG. Chromophore-assisted laser inactivation of cellular proteins. Methods Cell Biol. 1994;44:716–732. - PubMed
-
- Bentley D, Toroian-Raymond A. Disoriented pathfinding by pioneer neuron growth cones deprived of filopodia by cytochalasin treatment. Nature. 1986;323:712–715. - PubMed
-
- Birgbauer E, Dinsmore JH, Winckler B, Lander AD, Solomon F. Association of ezrin isoforms with the neuronal cytoskeleton. J Neurosci Res. 1991;30:232–241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

