Ras family GTPases control growth of astrocyte processes
- PMID: 10233170
- PMCID: PMC30489
- DOI: 10.1091/mbc.10.5.1665
Ras family GTPases control growth of astrocyte processes
Abstract
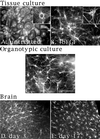
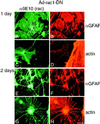
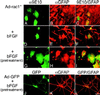
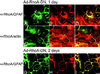
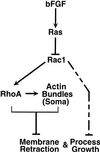
Astrocytes in neuron-free cultures typically lack processes, although they are highly process-bearing in vivo. We show that basic fibroblast growth factor (bFGF) induces cultured astrocytes to grow processes and that Ras family GTPases mediate these morphological changes. Activated alleles of rac1 and rhoA blocked and reversed bFGF effects when introduced into astrocytes in dissociated culture and in brain slices using recombinant adenoviruses. By contrast, dominant negative (DN) alleles of both GTPases mimicked bFGF effects. A DN allele of Ha-ras blocked bFGF effects but not those of Rac1-DN or RhoA-DN. Our results show that bFGF acting through c-Ha-Ras inhibits endogenous Rac1 and RhoA GTPases thereby triggering astrocyte process growth, and they provide evidence for the regulation of this cascade in vivo by a yet undetermined neuron-derived factor.
Figures





References
-
- Bignami A, Eng LF, Dahl D, Uyeda CT. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972;43:429–435. - PubMed
-
- Cameron RS, Rakic P. Glial cell lineage in the cerebral cortex: a review and synthesis. Glia. 1991;4:124–137. - PubMed
-
- Chen SY, Huff SY, Lai CC, Der CJ, Powers S. Ras-15A protein shares highly similar dominant-negative biological properties with Ras-17N and forms a stable, guanine-nucleotide resistant complex with CDC25 exchange factor. Oncogene. 1994;9:2691–2698. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

