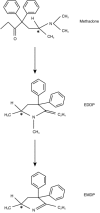
Methadone N-demethylation in human liver microsomes: lack of stereoselectivity and involvement of CYP3A4
- PMID: 10233205
- PMCID: PMC2014231
- DOI: 10.1046/j.1365-2125.1999.00921.x
Methadone N-demethylation in human liver microsomes: lack of stereoselectivity and involvement of CYP3A4
Abstract
Aims: To investigate the kinetics of CYP-mediated N-demethylation of methadone in human liver microsomes, and examine the role of stereoselectivity and CYP isoforms involved.
Methods: The kinetics of 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP) formation via N-demethylation of rac-, (R)- and (S)-methadone in human liver microsomes prepared from six liver samples were determined by h.p.l.c., and inhibition of metabolic function was studied using isoform-specific chemical inhibitors and monoclonal antibodies. Microsomes containing expressed CYP3A4, CYP2D6 and CYP2C19 were also used to examine the formation of EDDP.
Results: The V max, Km, and CLint values for the formation of EDDP from rac-, (R)- and (S)-methadone were in the ranges of 20-77 nmol mg-1 protein h-1, 125-252 microm, and 91-494 ml h-1 g-1 protein. Km and CLint values for (R)- and (S)-methadone were not statistically significantly different (P >0.05), while V max values for (S)-methadone were 15% (P=0.045) lower than for (R)-methadone. Expressed CYP3A4 and CYP2C19 showed similar reaction rates for both (R)- and (S)-methadone, while CYP2D6 did not catalyse this reaction. Selective chemical inhibitors of CYP3A (troleandomycin, ketoconazole) and monoclonal human CYP3A4 antibodies significantly inhibited (P<0.05) the formation of EDDP in a concentration dependent manner by up to 80%. Sulphaphenazole (CYP2C9) also significantly inhibited (P<0.05) EDDP formation (range 14-25%). There were no statistically significant differences in the inhibition observed between the three substrates. Selective inhibitors of CYP1A2 (furafylline), CYP2A6 (coumarin), CYP2C19 ((S)-mephenytoin), CYP2D6 (quinidine) and CYP2E1 (diethyldithiocarbamic acid sodium salt and monoclonal human CYP2E1 antibodies) had no significant (P >0.05) effect.
Conclusions: The N-demethylation of methadone in human liver microsomes is not markedly stereoselective, and is mediated mainly by CYP3A4 with the possible involvement of CYP2C9 and CYP2C19. Thus, the large interindividual variation reported for methadone pharmacokinetics may be due to variability in the expression of these CYP isoforms, and the reported stereoselectivity in the systemic clearance of methadone in vivo is not due to stereoselectivity in N-demethylation.
Figures
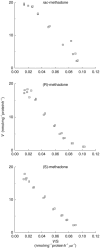
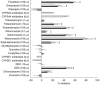
 ) (S)-methadone by human liver microsomes (n=3). Error bars indicate s.d. *Indicates statistically significant inhibition compared with control (P<0.05). No statistically significant differences (P0.05) were observed between rac-, (R)-, and (S)-methadone.
) (S)-methadone by human liver microsomes (n=3). Error bars indicate s.d. *Indicates statistically significant inhibition compared with control (P<0.05). No statistically significant differences (P0.05) were observed between rac-, (R)-, and (S)-methadone.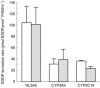
 ) (S)-methadone by microsomes from a human liver (HLS#5) and human lymphoblastoid cells containing the expressed individual CYP450 isoforms CYP3A4 and CYP2C19 (n=2). Error bars indicate s.d.
) (S)-methadone by microsomes from a human liver (HLS#5) and human lymphoblastoid cells containing the expressed individual CYP450 isoforms CYP3A4 and CYP2C19 (n=2). Error bars indicate s.d.Similar articles
-
Stereoselective metabolism of methadone N-demethylation by cytochrome P4502B6 and 2C19.Chirality. 2004 Jan;16(1):36-44. doi: 10.1002/chir.10303. Chirality. 2004. PMID: 14628297
-
Metabolism of methadone and levo-alpha-acetylmethadol (LAAM) by human intestinal cytochrome P450 3A4 (CYP3A4): potential contribution of intestinal metabolism to presystemic clearance and bioactivation.J Pharmacol Exp Ther. 2001 Sep;298(3):1021-32. J Pharmacol Exp Ther. 2001. PMID: 11504799
-
Enantiomeric metabolic interactions and stereoselective human methadone metabolism.J Pharmacol Exp Ther. 2007 Apr;321(1):389-99. doi: 10.1124/jpet.106.117580. Epub 2007 Jan 26. J Pharmacol Exp Ther. 2007. PMID: 17259447
-
Stereo-selective metabolism of methadone by human liver microsomes and cDNA-expressed cytochrome P450s: a reconciliation.Basic Clin Pharmacol Toxicol. 2011 Jan;108(1):55-62. doi: 10.1111/j.1742-7843.2010.00628.x. Epub 2010 Sep 2. Basic Clin Pharmacol Toxicol. 2011. PMID: 20825389 Free PMC article.
-
Metabolism of levo-alpha-Acetylmethadol (LAAM) by human liver cytochrome P450: involvement of CYP3A4 characterized by atypical kinetics with two binding sites.J Pharmacol Exp Ther. 2001 Apr;297(1):410-22. J Pharmacol Exp Ther. 2001. PMID: 11259570
Cited by
-
Contribution of the activities of CYP3A, CYP2D6, CYP1A2 and other potential covariates to the disposition of methadone in patients undergoing methadone maintenance treatment.Br J Clin Pharmacol. 2009 Jan;67(1):29-37. doi: 10.1111/j.1365-2125.2008.03312.x. Br J Clin Pharmacol. 2009. PMID: 19133059 Free PMC article.
-
A systems omics-based approach to decode substance use disorders and neuroadaptations.Neurosci Biobehav Rev. 2021 Nov;130:61-80. doi: 10.1016/j.neubiorev.2021.08.016. Epub 2021 Aug 17. Neurosci Biobehav Rev. 2021. PMID: 34411560 Free PMC article. Review.
-
ABCB1, CYP2B6, and CYP3A4 genetic polymorphisms do not affect methadone maintenance treatment in HCV-positive patients.Arh Hig Rada Toksikol. 2020 Dec 31;71(4):353-358. doi: 10.2478/aiht-2020-71-3378. Arh Hig Rada Toksikol. 2020. PMID: 33410778 Free PMC article.
-
Drug-Drug Interaction Studies of Esmethadone (REL-1017) Involving CYP3A4- and CYP2D6-Mediated Metabolism.Drugs R D. 2024 Mar;24(1):51-68. doi: 10.1007/s40268-023-00450-6. Epub 2023 Nov 27. Drugs R D. 2024. PMID: 38010591 Free PMC article. Clinical Trial.
-
Role of cytochrome P4502B6 in methadone metabolism and clearance.J Clin Pharmacol. 2013 Mar;53(3):305-13. doi: 10.1002/jcph.1. Epub 2013 Jan 7. J Clin Pharmacol. 2013. PMID: 23361846 Free PMC article. Clinical Trial.
References
-
- Kristensen K, Christensen CB, Christrup LL. The mu1, mu2, delta, kappa opioid receptor binding profiles of methadone stereoisomers and morphine. Life Sci. 1995;56:45–50. - PubMed
-
- Scott CC, Robbins EB, Chen KK. Pharmacologic comparison of the optical isomers of methadon. J Pharmacol Exp Ther. 1948;93:282–286. - PubMed
-
- Isbell H, Eisenman AJ. The addiction liability of some of the drugs of the methadon series. Fed Proc. 1948;7:162. - PubMed
-
- Kristensen K, Blemmer T, Angelo HR, et al. Stereoselective pharmacokinetics of methadone in chronic pain patients. Ther Drug Monit. 1996;18:221–227. - PubMed
-
- Eap CB, Cuendet C, Baumann P. Binding of d-methadone, l-methadone, and dl-methadone to proteins in plasma of healthy volunteers: role of the variants of alpha 1-acid glycoprotein. Clin Pharmacol Ther. 1990;47:338–346. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

