The radiation-attenuated schistosome vaccine induces high levels of protective immunity in the absence of B cells
- PMID: 10233674
- PMCID: PMC2326719
- DOI: 10.1046/j.1365-2567.1999.00661.x
The radiation-attenuated schistosome vaccine induces high levels of protective immunity in the absence of B cells
Abstract
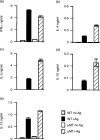
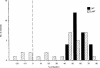
Radiation-attenuated cercariae of Schistosoma mansoni elicit consistently high levels of protective immunity in mice. The cell-mediated pulmonary effector mechanisms have been well characterized but the role of B cells and antibodies remains ill defined. We have compared the immune responses of B-cell-deficient (muMT) mice and their wild-type (WT) counterparts following exposure to the attenuated vaccine. Both groups mounted a T helper type 1 (Th1)-biased response in the skin-draining lymph nodes after vaccination. Interferon-gamma was the dominant cytokine secreted by airway leucocytes after challenge in both muMT and WT mice, but there was a somewhat greater Th2 component in the former animals. The cellular infiltrates observed in the airways, and the pulmonary effector foci, were of similar composition in the two groups although some large foci were present in the muMT mice. There was a marked dichotomy in the protection induced in muMT animals by a single vaccination, with two-thirds showing levels similar to their WT counterparts, demonstrating that cell-mediated mechanisms alone can provide adequate protection. The remaining muMT mice had a mean worm burden identical to that of their challenge controls. A possible explanation is that a proportion of the muMT animals have a genetic defect closely associated with the mu-heavy-chain locus on chromosome 12, which affects their ability to mount a protective cell-mediated response. Three vaccinations enhanced the immunity of WT animals, most likely by augmenting antibody-mediated mechanisms. In contrast, no enhancement was seen in muMT mice, suggesting that the cell-mediated response is not boosted by multiple exposures to attenuated larvae.
Figures

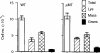


Similar articles
-
Optimal vaccination against Schistosoma mansoni requires the induction of both B cell- and IFN-gamma-dependent effector mechanisms.J Immunol. 1999 Jan 1;162(1):345-51. J Immunol. 1999. PMID: 9886405
-
Systems Biology Analysis of the Radiation-Attenuated Schistosome Vaccine Reveals a Role for Growth Factors in Protection and Hemostasis Inhibition in Parasite Survival.Front Immunol. 2021 Mar 11;12:624191. doi: 10.3389/fimmu.2021.624191. eCollection 2021. Front Immunol. 2021. PMID: 33777004 Free PMC article.
-
The generation of interferon-gamma-producing T lymphocytes in skin-draining lymph nodes, and their recruitment to the lungs, is associated with protective immunity to Schistosoma mansoni.Immunology. 1992 Feb;75(2):250-6. Immunology. 1992. PMID: 1532378 Free PMC article.
-
Immunity induced by the radiation-attenuated schistosome vaccine.Parasite Immunol. 2005 Jul-Aug;27(7-8):271-80. doi: 10.1111/j.1365-3024.2005.00764.x. Parasite Immunol. 2005. PMID: 16138848 Review.
-
Immune responses to the radiation-attenuated schistosome vaccine: what can we learn from knock-out mice?Immunol Lett. 1999 Jan;65(1-2):117-23. doi: 10.1016/s0165-2478(98)00134-5. Immunol Lett. 1999. PMID: 10065637 Review.
Cited by
-
The Road to Elimination: Current State of Schistosomiasis Research and Progress Towards the End Game.Front Immunol. 2022 May 3;13:846108. doi: 10.3389/fimmu.2022.846108. eCollection 2022. Front Immunol. 2022. PMID: 35592327 Free PMC article. Review.
-
Ultraviolet- attenuated cercariae of Schistosoma japonicum fail to effectively induce a Th1 response in spite of up-regulating expression of cytotoxicity-related genes in C57BL/6 mice.J Biomed Res. 2010 Jul;24(4):277-84. doi: 10.1016/S1674-8301(10)60039-5. J Biomed Res. 2010. PMID: 23554641 Free PMC article.
-
Why the radiation-attenuated cercarial immunization studies failed to guide the road for an effective schistosomiasis vaccine: A review.J Adv Res. 2015 May;6(3):255-67. doi: 10.1016/j.jare.2014.10.002. Epub 2014 Oct 20. J Adv Res. 2015. PMID: 26257924 Free PMC article. Review.
-
Signaling via interleukin-4 receptor alpha chain is required for successful vaccination against schistosomiasis in BALB/c mice.Infect Immun. 2001 Jan;69(1):228-36. doi: 10.1128/IAI.69.1.228-236.2001. Infect Immun. 2001. PMID: 11119510 Free PMC article.
-
Schistosomiasis in the People's Republic of China: prospects and challenges for the 21st century.Clin Microbiol Rev. 2001 Apr;14(2):270-95. doi: 10.1128/CMR.14.2.270-295.2001. Clin Microbiol Rev. 2001. PMID: 11292639 Free PMC article. Review.
References
-
- Coulson PS. The radiation-attenuated vaccine against schistosomes in animal models: paradigm for a human vaccine. Adv Parasitol. 1997;39:271. - PubMed
-
- Smythies LE, Betts C, Coulson PS, Dowling MA, Wilson RA. Kinetics and mechanism of effector focus formation in the lungs of mice vaccinated with irradiated cercariae of Schistosoma mansoni. Parasite Immunol. 1996;18:359. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

