Surface-expressed invariant chain (CD74) is required for internalization of human leucocyte antigen-DR molecules to early endosomal compartments
- PMID: 10233730
- PMCID: PMC2326754
- DOI: 10.1046/j.1365-2567.1999.00676.x
Surface-expressed invariant chain (CD74) is required for internalization of human leucocyte antigen-DR molecules to early endosomal compartments
Abstract
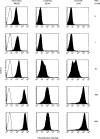
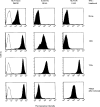
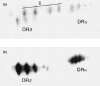
Transport of major histocompatibility complex (MHC) class II molecules to the endocytic route is directed by the associated invariant chain (Ii). In the endocytic pathway, Ii is proteolytically cleaved and, upon removal of residual Ii fragments, class II alpha beta dimers are charged with antigenic peptide and recognized by CD4+ T cells. Although distinct peptide-loading compartments such as MIIC (MHC class II loading compartment) and CIIV (MHC class II vesicles) have been characterized in different cells, there is growing evidence of a multitude of subcellular compartments in which antigenic peptide loading takes place. We employed a physiological cellular system in which surface Ii (CD74) and surface human leucocyte antigen (HLA)-DR were induced either alone or in combination. This was achieved by transient exposure of HT-29 cells to recombinant interferon-gamma (rIFN-gamma). Using distinct cellular variants, we showed that: (i) the majority of Ii molecules physically associate on the cell membrane with class II dimers to form DR alpha beta:Ii complexes; (ii) the presence of surface Ii is a prerequisite for the rapid uptake of HLA-DR-specific monoclonal antibodies into early endosomes because only the surface DR+/Ii+ phenotype, and not the DR+/Ii- variant, efficiently internalizes; and (iii) the HLA-DR:Ii complexes are targeted to early endosomes, as indicated by co-localization with the GTPase, Rab5, and endocytosed bovine serum albumin. Internalization of HLA-DR:Ii complexes, accommodation of peptides by DR alphabeta heterodimers in early endosomes and recycling to the cell surface may be a mechanism used to increase the peptide repertoire that antigen-presenting cells display to MHC class II-restricted T cells.
Figures




Similar articles
-
Interaction of HLA-DR and CD74 at the cell surface of antigen-presenting cells by single particle image analysis.FASEB J. 2012 Dec;26(12):4886-96. doi: 10.1096/fj.12-211466. Epub 2012 Aug 13. FASEB J. 2012. PMID: 22889831
-
Major histocompatibility complex class II compartments in human B lymphoblastoid cells are distinct from early endosomes.J Exp Med. 1995 Aug 1;182(2):325-34. doi: 10.1084/jem.182.2.325. J Exp Med. 1995. PMID: 7629497 Free PMC article.
-
Attenuation of HLA-DR expression by mononuclear phagocytes infected with Mycobacterium tuberculosis is related to intracellular sequestration of immature class II heterodimers.J Immunol. 1998 Nov 1;161(9):4882-93. J Immunol. 1998. PMID: 9794422
-
Endosomal localization of MHC class II-invariant chain complexes.Immunol Res. 1994;13(4):244-52. doi: 10.1007/BF02935616. Immunol Res. 1994. PMID: 7616052 Review.
-
MHC II and the endocytic pathway: regulation by invariant chain.Scand J Immunol. 2009 Sep;70(3):184-93. doi: 10.1111/j.1365-3083.2009.02301.x. Scand J Immunol. 2009. PMID: 19703008 Review.
Cited by
-
Immunophenotyping of the cluster of differentiation 74, migration inhibitory factor, and cluster of differentiation 44 expression on human breast cancer-derived cell lines.Int J Health Sci (Qassim). 2019 Mar-Apr;13(2):17-24. Int J Health Sci (Qassim). 2019. PMID: 30983941 Free PMC article.
-
Hepatitis C virus-mediated inhibition of cathepsin S increases invariant-chain expression on hepatocyte surface.J Virol. 2012 Sep;86(18):9919-28. doi: 10.1128/JVI.00388-12. Epub 2012 Jul 3. J Virol. 2012. PMID: 22761382 Free PMC article.
-
An Epstein-Barr Virus (EBV) mutant with enhanced BZLF1 expression causes lymphomas with abortive lytic EBV infection in a humanized mouse model.J Virol. 2012 Aug;86(15):7976-87. doi: 10.1128/JVI.00770-12. Epub 2012 May 23. J Virol. 2012. PMID: 22623780 Free PMC article.
-
Gene expression in Atlantic salmon skin in response to infection with the parasitic copepod Lepeophtheirus salmonis, cortisol implant, and their combination.BMC Genomics. 2012 Apr 5;13:130. doi: 10.1186/1471-2164-13-130. BMC Genomics. 2012. PMID: 22480234 Free PMC article.
-
The secret life of viral entry glycoproteins: moonlighting in immune evasion.PLoS Pathog. 2013;9(5):e1003258. doi: 10.1371/journal.ppat.1003258. Epub 2013 May 16. PLoS Pathog. 2013. PMID: 23696729 Free PMC article. Review. No abstract available.
References
-
- Nordeng TW, Bakke O. The biological role of invariant chain (Ii) in MHC class II antigen presentation. Immunol Lett. 1994;43:47. - PubMed
-
- Cresswell P. Assembly, transport and function of MHC class II molecules. Annu Rev Immunol. 1994;12:259. - PubMed
-
- Kvist S, Wiman K, Claesson L, Peterson PA, Dobberstein B. Membrane insertion and oligomeric assembly of HLA-DR histocompatibility antigens. Cell. 1982;29:61. - PubMed
-
- Bakke O, Dobberstein B. MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell. 1990;63:707. - PubMed
-
- Lotteau V, Teyton L, Peleraux A, et al. Intracellular transport of class II MHC molecules directed by invariant chain. Nature. 1990;348:600. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

