Interleukin-4 and interferon-gamma synergistically increase secretory component gene expression, but are additive in stimulating secretory immunoglobulin A release by Calu-3 airway epithelial cells
- PMID: 10233739
- PMCID: PMC2326789
- DOI: 10.1046/j.1365-2567.1999.00731.x
Interleukin-4 and interferon-gamma synergistically increase secretory component gene expression, but are additive in stimulating secretory immunoglobulin A release by Calu-3 airway epithelial cells
Abstract
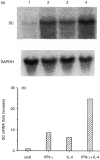
Interleukin-4 (IL-4) and interferon-gamma (IFN-gamma) synergize to express polymeric immunoglobulin receptor (pIgR) but their combined effect, and that of IL-4 alone, on secretory immunoglobulin A (sIgA) release is unknown. Recently, we have developed an airway epithelial cell model that allows assessment of the integrated effect of a stimulus on pIgR gene and protein expression and sIgA release. With this model we show here that IL-4 and IFN-gamma dose-dependently increased pIgR mRNA and protein expression, and sIgA release. IFN-gamma and IL-4 induced similar maximal expression of pIgR, but IFN-gamma enhanced sIgA release more than IL-4. When added together, IL-4 and IFN-gamma synergistically increased pIgR mRNA and protein expression, but sIgA release was stimulated in an additive manner. Thus, IL-4 and IFN-gamma may be implicated in the increase of sIgA levels as found in mucosal inflammatory diseases. In addition, our results indicate that transport and release of empty pIgR is subject to regulatory mechanisms different from those of pIgR with bound dimeric IgA.
Figures



References
-
- Childers NK, Bruce MG, McGhee JR. Molecular mechanisms of immunoglobulin A defense. Annu Rev Microbiol. 1989;43:503. - PubMed
-
- Heremans JF. Immunoglobulin A. In: Sela M, editor. The Antigens. Vol. 2. New York: Academic Press; 1974. p. 365.
-
- Mostov KE. Transepithelial transport of immunoglobulins. Annu Rev Immunol. 1994;12:63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

