Molecular evolution of the metaplasia-dysplasia-adenocarcinoma sequence in the esophagus
- PMID: 10233832
- PMCID: PMC1866556
- DOI: 10.1016/S0002-9440(10)65346-1
Molecular evolution of the metaplasia-dysplasia-adenocarcinoma sequence in the esophagus
Abstract
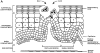
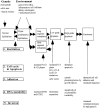
The incidence of adenocarcinoma of the esophagus has been increasing in developing countries over the last three decades and probably reflects a genuine increase in the incidence of its recognized precursor lesion, Barrett's metaplasia. Despite advances in multimodality therapy, the prognosis for invasive esophageal adenocarcinoma is poor. An improved understanding of the molecular biology of this disease may allow improved diagnosis, therapy, and prognosis. We focus on recent developments in the molecular and cell biology of Barrett's metaplasia, a heterogeneous lesion affecting the transitional zone of the gastro-esophageal junction whose associated molecular alterations may vary both in nature and temporally. Early premalignant clones produce biological and genetic heterogeneity as seen by multiple p53 mutations, p16 mutations, aneuploidy, and abnormal methylation resulting in stepwise changes in differentiation, proliferation, and apoptosis, allowing disease progression under selective pressure. Abnormalities in expression of growth factors of the epidermal growth factor family and cell adhesion molecules, especially cadherin/catenin complexes, may occur early in invasion. Exploitation of these molecular events may lead to a more appropriate diagnosis and understanding of these lesions in the future.
Figures



References
-
- Spechler SJ: Barrett’s esophagus. Sem Oncol 1994, 21:431-437 - PubMed
-
- Winter C, Jr, Spurling TJ, Chobabian SJ, Curtis DJ, Esposito RL, Hacker JF, III, Johnston DA, Cruess DF, Cotelingam JD, Gurney MS, Cattau EL, Jr: Barrett’s esophagus: a prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology 1987, 92:118-124 - PubMed
-
- Prach AT, MacDonald TA, Hopwood DA, Johnston DA: Increasing incidence of Barrett’s oesophagus: education, enthusiasm or epidemiology. Lancet 1997, 350:933 - PubMed
-
- Beynon J, Pye JK, Howell P, Nehra D: Assessment of combined bile acid and pH profiles using an automated sampling device in gastro-oesophageal reflux disease. Br J Surg 1998, 85:134-137 - PubMed
-
- Romero Y, Cameron AJ, Locke GR, Schaid DJ, Melton LJ, Slexak J: Familial gastroesophageal reflux: association with Barrett’s esophagus and adenocarcinoma. Gastroenterology 1997, 110:456 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

