Mechanisms of enhanced lung injury during sepsis
- PMID: 10233844
- PMCID: PMC1866577
- DOI: 10.1016/S0002-9440(10)65358-8
Mechanisms of enhanced lung injury during sepsis
Abstract
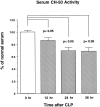
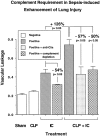
A major complication in sepsis is progressively impaired lung function and susceptibility to intrapulmonary infection. Why sepsis predisposes the lung to injury is not clear. In the current studies, rats were rendered septic by cecal ligation/puncture and evaluated for increased susceptibility to injury after a direct pulmonary insult (deposition of IgG immune complexes or airway instillation of lipopolysaccharide). By itself, cecal ligation/puncture did not produce evidence of lung injury. However, after a direct pulmonary insult, lung injury in septic animals was significantly enhanced. Enhanced lung injury was associated with increased accumulation of neutrophils in lung, enhanced production of CXC chemokines (but not tumor necrosis factor-alpha) in bronchoalveolar lavage fluids, and increased expression of lung vascular intercellular adhesion molecule-1 (ICAM-1). Complement depletion or treatment with anti-C5a abolished all evidence of enhanced lung injury in septic animals. When stimulated in vitro, bronchoalveolar lavage macrophages from septic animals had greatly enhanced CXC chemokine responses as compared with macrophages from sham-operated animals or from septic animals that had been complement depleted. These data indicate that the septic state causes priming of lung macrophages and suggest that enhanced lung injury in the septic state is complement dependent and related to increased production of CXC chemokines.
Figures






References
-
- Young LS: Gram-negative sepsis. Mandell GL Douglas SD Bennett JC eds. Principles and Practice of Infectious Diseases. 1979, :pp 571-608 John Wiley and Sons, New York
-
- Bone RC: The sepsis syndrome: definition and general approach to management. Clin Chest Med 1996, 17:175-181 - PubMed
-
- Kaplan RL, Sahn SA, Petty TL: Incidence and outcome of respiratory distress syndrome in gram-negative sepsis. Arch Intern Med 1979, 139:867-869 - PubMed
-
- Moore FA, Moore EE, Read RA: Postinjury multiple organ failure: role of extrathoracic injury and sepsis in adult respiratory distress syndrome. New Horizons 1993, 1:538-549 - PubMed
-
- Polk HC, Shields CL: Remote organ failure: a valid sign of occult intra-abdominal infection. Surgery 1977, 81:310-331 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

