Vascular endothelial growth factor and basic fibroblast growth factor induce expression of CXCR4 on human endothelial cells: In vivo neovascularization induced by stromal-derived factor-1alpha
- PMID: 10233851
- PMCID: PMC1866563
- DOI: 10.1016/s0002-9440(10)65365-5
Vascular endothelial growth factor and basic fibroblast growth factor induce expression of CXCR4 on human endothelial cells: In vivo neovascularization induced by stromal-derived factor-1alpha
Abstract
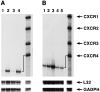
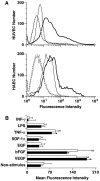
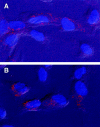
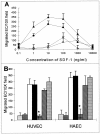
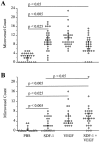
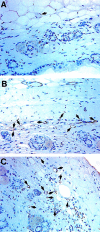
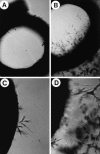
The contribution of chemokines toward angiogenesis is currently a focus of intensive investigation. Certain members of the CXC chemokine family can induce bovine capillary endothelial cell migration in vitro and corneal angiogenesis in vivo, and apparently act via binding to their receptors CXCR1 and CXCR2. We used an RNAse protection assay that permitted the simultaneous detection of mRNA for various CXC chemokine receptors in resting human umbilical vein endothelial cells (HUVECs) and detected low levels of only CXCR4 mRNA. Stimulation of HUVECs with vascular endothelial growth factor (VEGF) or basic fibroblast growth factor (bFGF) up-regulated levels of only CXCR4 mRNA. CXCR4 specifically binds the chemokine stromal-derived factor-1alpha (SDF-1alpha). Competitive binding studies using 125I-labeled SDF-1alpha with Scatchard analysis indicated that VEGF or bFGF induced an average number of approximately 16,600 CXCR4 molecules per endothelial cell, with a Kd = 1.23 x 10(-9) mol/L. These receptors were functional as HUVECs and human aorta endothelial cells (HAECs) migrated toward SDF-1alpha. Although SDF-1alpha-induced chemotaxis was inhibited by the addition of a neutralizing monoclonal CXCR4 antibody, endothelial chemotaxis toward VEGF was not altered; therefore, the angiogenic effect of VEGF is independent of SDF-1alpha. Furthermore, subcutaneous SDF-1alpha injections into mice induced formation of local small blood vessels that was accompanied by leukocytic infiltrates. To test whether these effects were dependent on circulating leukocytes, we successfully obtained SDF-1alpha-induced neovascularization from cross sections of leukocyte-free rat aorta. Taken together, our data indicate that SDF-1alpha acts as a potent chemoattractant for endothelial cells of different origins bearing CXCR4 and is a participant in angiogenesis that is regulated at the receptor level by VEGF and bFGF.
Figures

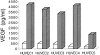

References
-
- Hanahan D, Folkman J: Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 86:353-364 - PubMed
-
- Ellis LM, Fidler IJ: Angiogenesis and metastasis. Eur J Cancer 1996, 32A:2451-2460 - PubMed
-
- Folkman J: Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature Med 1995, 1:27-31 - PubMed
-
- Auerbach W, Auerbach R: Angiogenesis inhibition: a review. Pharmacol Ther 1994, 63:265-311 - PubMed
-
- Bussolino F, Albini A, Camussi G, Presta M, Viglietto G, Ziche M, Persico G: Role of soluble mediators in angiogenesis. Eur J Cancer 1996, 32A:2401-2412 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

