Herpesvirus ateles gene product Tio interacts with nonreceptor protein tyrosine kinases
- PMID: 10233922
- PMCID: PMC112504
- DOI: 10.1128/JVI.73.6.4631-4639.1999
Herpesvirus ateles gene product Tio interacts with nonreceptor protein tyrosine kinases
Abstract
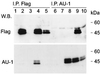
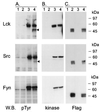
Herpesvirus ateles is a gamma-2-herpesvirus which naturally infects spider monkeys (Ateles spp.) and causes malignant lymphoproliferative disorders in various other New World primates. The genomic sequence of herpesvirus ateles strain 73 revealed a close relationship to herpesvirus saimiri, with a high degree of variability within the left terminus of the coding region. A spliced mRNA transcribed from this region was detected in New World monkey T-cell lines transformed by herpesvirus ateles in vitro or derived from T cells of infected Saguinus oedipus. The encoded viral protein, termed Tio, shows restricted homology to the oncoprotein StpC and to the tyrosine kinase-interacting protein Tip, two gene products responsible for the T-cell-transforming and oncogenic phenotype of herpesvirus saimiri group C strains. Tio was detectable in lysates of the transformed T lymphocytes. Dimer formation was observed after expression of recombinant Tio. After cotransfection, Tio was phosphorylated in vivo by the protein tyrosine kinases Lck and Src and less efficiently by Fyn. Stable complexes of these Src family kinases with the viral protein were detected in lysates of the transfected cells. Binding analyses indicated a direct interaction of Tio with the SH3 domains of Lyn, Hck, Lck, Src, Fyn, and Yes. In addition, tyrosine-phosphorylated Tio bound to the SH2 domains of Lck, Src, or Fyn. Thus, herpesvirus ateles-encoded Tio may contribute to viral T-cell transformation by influencing the function of Src family kinases.
Figures






References
-
- Albrecht, J. C., and B. Fleckenstein. Unpublished data. GenBank accession no. AF083424.
-
- Biesinger, B. Unpublished data.
-
- Biesinger B, Trimble J J, Desrosiers R C, Fleckenstein B. The divergence between two oncogenic Herpesvirus saimiri strains in a genomic region related to the transforming phenotype. Virology. 1990;176:505–514. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

