Encephalopsin: a novel mammalian extraretinal opsin discretely localized in the brain
- PMID: 10234000
- PMCID: PMC6782724
- DOI: 10.1523/JNEUROSCI.19-10-03681.1999
Encephalopsin: a novel mammalian extraretinal opsin discretely localized in the brain
Abstract
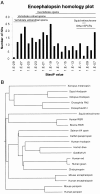
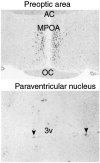
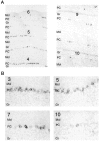
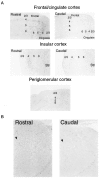
We have identified a mammalian opsin, encephalopsin, that shows strong and specific expression in the brain. Encephalopsin defines a new family of opsins and shows highest homology to vertebrate retinal and pineal opsins. Encephalopsin is highly expressed in the preoptic area and paraventricular nucleus of the hypothalamus, both regions implicated in encephalic photoreception in nonmammalian vertebrates. In addition, encephalopsin shows highly patterned expression in other regions of the brain, being enriched in selected regions of the cerebral cortex, cerebellar Purkinje cells, a subset of striatal neurons, selected thalamic nuclei, and a subset of interneurons in the ventral horn of the spinal cord. Rostrocaudal gradients of encephalopsin expression are present in the cortex, cerebellum, and striatum. Radial stripes of encephalopsin expression are seen in the cerebellum. In the cortex and cerebellum, encephalopsin expression is considerably higher and more highly patterned in the adult than in the neonate. Encephalopsin is the first putative extraocular opsin identified in mammals and may play a role in encephalic photoreception.
Figures





References
-
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. - PubMed
-
- Campbell SS, Murphy PJ. Extraocular circadian phototransduction in humans. Science. 1998;279:396–399. - PubMed
-
- Cepko CL. The patterning and onset of opsin expression in vertebrate retinae. Curr Opin Neurobiol. 1996;6:542–546. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
