Spontaneous activity in developing turtle retinal ganglion cells: pharmacological studies
- PMID: 10234019
- PMCID: PMC6782712
- DOI: 10.1523/JNEUROSCI.19-10-03874.1999
Spontaneous activity in developing turtle retinal ganglion cells: pharmacological studies
Abstract
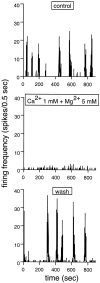
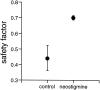
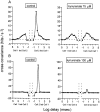
Extracellular recordings were obtained from the ganglion cell (GC) layer during correlated spontaneous bursting activity (SBA) in the immature turtle retina. Pharmacological agents were bath-applied, and their effects on burst and correlation parameters were determined. SBA requires synaptic transmission. It was blocked in the presence of curare and mecamylamine, two cholinergic nicotinic antagonists, and enhanced with neostigmine, a cholinesterase inhibitor. SBA was profoundly inhibited during blockade of glutamatergic receptors with the broad spectrum antagonist kynurenate and it vanished with 6,7-dinitroquinoxaline-2-3-dione (DNQX) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), two AMPA/kainate receptor antagonists. Blockade of NMDA receptors with D(-)-2-amino-5-phosphonopentanoic acid (D-AP-5) led only to a modest reduction in SBA. Blockade of GABAA receptors with bicuculline prolonged the duration of the bursts. Inhibition of GABA uptake with nipecotic acid led to a decrease in burst rate. Blockade of K+ channels with cesium (Cs+) and tetraethylammonium (TEA) led to a dramatic decrease in excitability. Burst propagation between neighboring GCs was reduced by K+ channel blockade. Gap junction blockade had no consistent effect on bursts or correlation parameters. None of these drugs had a strong effect on the refractory period between bursts. We conclude that correlated SBA in immature turtle GCs requires both cholinergic nicotinic and glutamatergic (mainly through AMPA/kainate receptors) synaptic transmission. GABAergic activity modulates the intensity and the duration of the bursts. Extracellular K+ is involved in lateral activity propagation and increases retinal excitability, which may be required for burst generation.
Figures







Similar articles
-
Physiological unmasking of new glutamatergic pathways in the dentate gyrus of hippocampal slices from kainate-induced epileptic rats.J Neurophysiol. 1998 Jan;79(1):418-29. doi: 10.1152/jn.1998.79.1.418. J Neurophysiol. 1998. PMID: 9425210
-
Spontaneous oscillatory activity of starburst amacrine cells in the mouse retina.J Neurophysiol. 2005 Sep;94(3):1770-80. doi: 10.1152/jn.00279.2005. Epub 2005 May 25. J Neurophysiol. 2005. PMID: 15917322
-
Potentiation of L-type calcium channels reveals nonsynaptic mechanisms that correlate spontaneous activity in the developing mammalian retina.J Neurosci. 2001 Nov 1;21(21):8514-22. doi: 10.1523/JNEUROSCI.21-21-08514.2001. J Neurosci. 2001. PMID: 11606640 Free PMC article.
-
Physiological effects of sustained blockade of excitatory synaptic transmission on spontaneously active developing neuronal networks--an inquiry into the reciprocal linkage between intrinsic biorhythms and neuroplasticity in early ontogeny.Neurosci Biobehav Rev. 2002 Mar;26(2):127-85. doi: 10.1016/s0149-7634(01)00062-8. Neurosci Biobehav Rev. 2002. PMID: 11856557 Review.
-
The role of early neural activity in the maturation of turtle retinal function.J Anat. 2001 Oct;199(Pt 4):375-83. doi: 10.1046/j.1469-7580.2001.19940375.x. J Anat. 2001. PMID: 11693298 Free PMC article. Review.
Cited by
-
An Evolutionarily Conserved Mechanism for Activity-Dependent Visual Circuit Development.Front Neural Circuits. 2016 Oct 21;10:79. doi: 10.3389/fncir.2016.00079. eCollection 2016. Front Neural Circuits. 2016. PMID: 27818623 Free PMC article. Review.
-
Developmental modulation of retinal wave dynamics: shedding light on the GABA saga.J Neurosci. 2003 Aug 20;23(20):7621-9. doi: 10.1523/JNEUROSCI.23-20-07621.2003. J Neurosci. 2003. PMID: 12930801 Free PMC article.
-
On the Role of LGN/V1 Spontaneous Activity as an Innate Learning Pattern for Visual Development.Front Physiol. 2021 Oct 27;12:695431. doi: 10.3389/fphys.2021.695431. eCollection 2021. Front Physiol. 2021. PMID: 34776991 Free PMC article.
-
Non-cell-autonomous factor induces the transition from excitatory to inhibitory GABA signaling in retina independent of activity.Proc Natl Acad Sci U S A. 2010 Dec 21;107(51):22302-7. doi: 10.1073/pnas.1008775108. Epub 2010 Dec 6. Proc Natl Acad Sci U S A. 2010. PMID: 21135238 Free PMC article.
-
Intrinsic and Synaptic Properties Shaping Diverse Behaviors of Neural Dynamics.Front Comput Neurosci. 2020 Apr 21;14:26. doi: 10.3389/fncom.2020.00026. eCollection 2020. Front Comput Neurosci. 2020. PMID: 32372936 Free PMC article.
References
-
- Ariel M, Adolph AR. Neurotransmitter inputs to directionally sensitive turtle retinal ganglion cells. J Neurophysiol. 1985;54:1123–1143. - PubMed
-
- Backman SB, Stein RD, Blank DW, Collier B, Polosa C. Different properties of the bradycardia produced by neostigmine and edrophonium in the cat. Can J Anaesth. 1996;43:731–740. - PubMed
-
- Brivanlou IH, Warland DK, Meister M. Mechanisms of concerted firing among retinal ganglion cells. Neuron. 1998;20:527–539. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
