Genesis, neurotrophin responsiveness, and apoptosis of a pronounced direct connection between the two eyes of the chick embryo: a natural error or a meaningful developmental event?
- PMID: 10234021
- PMCID: PMC6782693
- DOI: 10.1523/JNEUROSCI.19-10-03900.1999
Genesis, neurotrophin responsiveness, and apoptosis of a pronounced direct connection between the two eyes of the chick embryo: a natural error or a meaningful developmental event?
Abstract
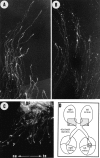
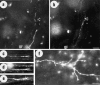
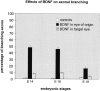
Unilateral intraocular injections of either of two fluorescent carbocyanine dyes into the embryonic chick eye resulted in both retrograde staining of ganglion cells (GCs) in the eye contralateral to site of injection and anterograde labeling of axons whose cell bodies were located within the injected eye. This prominent retino-retinal projection formed by thousands of GCs having a nasal origin and temporal termination appeared at embryonic day 6 (E6), attained its maximum intensity at E13-E14, and gradually disappeared until E18. The axonal growth cones ended superficially and never penetrated deeper layers of the retina. Treatment of the projection with BDNF resulted in massive terminal branching of the axons within deeper layers of the target retina. Double injection into the eye and the isthmo-optic nucleus showed a concomitant ingrowth of axons in the contralateral retina. Individual GCs died between E9 and E13, but massive apoptotic cell death was mainly monitored at E14 and later. Disintegrated cells showed typical images of apoptosis. Because degenerating cells were prelabeled with the membranophilic fluorescent carbocyanine dye, their death allowed the concomitant visualization of phagocytosing cells, too. Radial Müller glia were the only class of cells observed to become phagocytotic between E9 and E16. These cells became replaced exclusively with microglial cells from E17 on. The results suggest that the topologically restricted retino-retinal projection may have some developmental significance rather than representing a massive erroneous projection. Most likely, the projection may serve as a "template" to guide centrifugal isthmo-optic axons into the retina.
Figures









Similar articles
-
The developing chick isthmo-optic nucleus forms a transient efferent projection to the optic tectum.Neurosci Lett. 1990 Jun 8;113(3):241-6. doi: 10.1016/0304-3940(90)90591-v. Neurosci Lett. 1990. PMID: 2381560
-
Development of two morphological types of retinopetal fibers in chick embryos, as shown by the diffusion along axons of a carbocyanine dye in the fixed retina.J Comp Neurol. 1990 Oct 15;300(3):405-21. doi: 10.1002/cne.903000310. J Comp Neurol. 1990. PMID: 2266193
-
Axonal arborization in the developing chick retinotectal system.J Comp Neurol. 1987 Jul 1;261(1):155-64. doi: 10.1002/cne.902610114. J Comp Neurol. 1987. PMID: 3624542
-
Spatial arrangement of radial glia and ingrowing retinal axons in the chick optic tectum during development.Brain Res Dev Brain Res. 1989 Jan 1;45(1):15-27. doi: 10.1016/0165-3806(89)90003-5. Brain Res Dev Brain Res. 1989. PMID: 2917409
-
Prenatal development of the optic projection in albino and hooded rats.Brain Res. 1983 Jan;282(2):149-68. doi: 10.1016/0165-3806(83)90093-7. Brain Res. 1983. PMID: 6831237
Cited by
-
Retinal ganglion cell interactions shape the developing mammalian visual system.Development. 2020 Dec 7;147(23):dev196535. doi: 10.1242/dev.196535. Development. 2020. PMID: 33288502 Free PMC article. Review.
-
Experience-dependent and independent binocular correspondence of receptive field subregions in mouse visual cortex.Cereb Cortex. 2014 Jun;24(6):1658-70. doi: 10.1093/cercor/bht027. Epub 2013 Feb 6. Cereb Cortex. 2014. PMID: 23389996 Free PMC article.
-
Distribution of scotoma pattern related to chiasmal lesions with special reference to anterior junction syndrome.Graefes Arch Clin Exp Ophthalmol. 2004 Jun;242(6):468-77. doi: 10.1007/s00417-004-0863-5. Epub 2004 Apr 7. Graefes Arch Clin Exp Ophthalmol. 2004. PMID: 15069563
-
Efferent influences on the bioelectrical activity of the retina in primates.Doc Ophthalmol. 2017 Feb;134(1):57-73. doi: 10.1007/s10633-016-9567-5. Epub 2016 Dec 28. Doc Ophthalmol. 2017. PMID: 28032236 Review.
-
Müller glia and phagocytosis of cell debris in retinal tissue.J Anat. 2017 Oct;231(4):471-483. doi: 10.1111/joa.12653. Epub 2017 Jul 10. J Anat. 2017. PMID: 28695619 Free PMC article. Review.
References
-
- Alvarez-Bolado G, Schwarz M, Gruss P. PAX-2 in the chiasm. Cell Tissue Res. 1997;290:197–200. - PubMed
-
- Apkarian P, Eckhardt PG, van-Schooneveld MJ. Detection of optic pathway misrouting in the human albino neonate. Neuropediatrics. 1991;22:211–215. - PubMed
-
- Bohn RC, Stelzner DJ. The aberrant retino-retinal projection during optic nerve regeneration in the frog. I. Time course of formation and cells of origin. J Comp Neurol. 1981a;196:605–620. - PubMed
-
- Bohn RC, Stelzner DJ. The aberrant retino-retinal projection during optic nerve regeneration in the frog. II. Anterograde labelling with horseradish peroxidase. J Comp Neurol. 1981b;196:621–632. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
