Differential regulation of the expression of corticotropin-releasing factor receptor type 2 (CRF2) in hypothalamus and amygdala of the immature rat by sensory input and food intake
- PMID: 10234028
- PMCID: PMC3139461
- DOI: 10.1523/JNEUROSCI.19-10-03982.1999
Differential regulation of the expression of corticotropin-releasing factor receptor type 2 (CRF2) in hypothalamus and amygdala of the immature rat by sensory input and food intake
Abstract
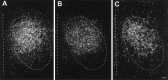
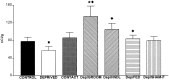
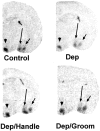
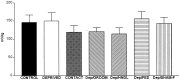
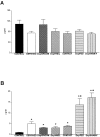
The physiological consequences of activating corticotropin-releasing factor receptor type 2 (CRF2) are not fully understood. The neuroanatomic distribution of this CRF receptor family member is consistent with roles in mediating the actions of CRF and similar ligands on food intake control and integrative aspects of stress-related behaviors. However, CRF2 expression in the adult rat is not influenced by stress, corticosterone (CORT), or food intake. In immature rat we have demonstrated striking downregulation of CRF2mRNA in hypothalamic ventromedial nucleus (VMH) after 24 hr of maternal deprivation, a paradigm consisting of both physiological/psychological stress and food deprivation. The current study aimed to distinguish which element or elements of maternal deprivation govern CRF2mRNA expression by isolating the effects of food intake and discrete maternal sensory cues on CRF2mRNA levels in VMH and in reciprocally communicating amygdala nuclei. In maternally deprived pups, CRF2mRNA levels in VMH and basomedial (BMA) and medial (MEA) amygdala nuclei were 62, 72, and 102% of control levels, respectively. Sensory inputs of grooming and handling as well as of the pups' own suckling activity-but not food intake-fully restored CRF2mRNA expression in VMH. In contrast, all manipulations tended to increase CRF2mRNA levels in BMA of maternally deprived rats, and surrogate grooming increased CRF2mRNA expression significantly above that of nondeprived controls. CRF2mRNA expression was not influenced significantly by plasma adrenocorticotropic hormone (ACTH) and CORT levels. Thus, in the immature rat, (1) CRF2 expression is regulated differentially in hypothalamic and amygdala regions, and (2) CRF2mRNA levels in VMH are governed primarily by maternal or suckling-derived sensory input rather than food intake or peripheral stress hormones. These findings indicate a region-specific regulation of CRF2mRNA, supporting the participation of the receptor in neurochemically defined circuits integrating sensory cues to influence specific behavioral and visceral functions.
Figures

References
-
- Baram TZ, Lerner SP. Ontogeny of corticotropin releasing hormone gene expression in rat hypothalamus—comparison with somatostatin. Int J Dev Neurosci. 1991;9:473–478. - PubMed
-
- Behan DP, Heinrichs SC, Troncoso JC, Liu XJ, Kawas CH, Ling N, De Souza EB. Displacement of corticotropin releasing factor from its binding protein as a possible treatment for Alzheimer’s disease. Nature. 1995;378:284–287. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
