5-HT1B receptor-mediated presynaptic inhibition of retinal input to the suprachiasmatic nucleus
- PMID: 10234032
- PMCID: PMC6782735
- DOI: 10.1523/JNEUROSCI.19-10-04034.1999
5-HT1B receptor-mediated presynaptic inhibition of retinal input to the suprachiasmatic nucleus
Abstract
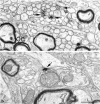
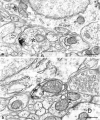
The suprachiasmatic nucleus (SCN) receives glutamatergic afferents from the retina and serotonergic afferents from the midbrain, and serotonin (5-HT) can modify the response of the SCN circadian oscillator to light. 5-HT1B receptor-mediated presynaptic inhibition has been proposed as one mechanism by which 5-HT modifies retinal input to the SCN (Pickard et al., 1996). This hypothesis was tested by examining the subcellular localization of 5-HT1B receptors in the mouse SCN using electron microscopic immunocytochemical analysis with 5-HT1B receptor antibodies and whole-cell patch-clamp recordings from SCN neurons in hamster hypothalamic slices. 5-HT1B receptor immunostaining was observed associated with the plasma membrane of retinal terminals in the SCN. 1-[3-(Trifluoromethyl)phenyl]-piperazine HCl (TFMPP), a 5-HT1B receptor agonist, reduced in a dose-related manner the amplitude of glutamatergic EPSCs evoked by stimulating selectively the optic nerve. Selective 5-HT1A or 5-HT7 receptor antagonists did not block this effect. Moreover, in cells demonstrating an evoked EPSC in response to optic nerve stimulation, TFMPP had no effect on the amplitude of inward currents generated by local application of glutamate. The effect of TFMPP on light-induced phase shifts was also examined using 5-HT1B receptor knock-out mice. TFMPP inhibited behavioral responses to light in wild-type mice but was ineffective in inhibiting light-induced phase shifts in 5-HT1B receptor knock-out mice. The results indicate that 5-HT can reduce retinal input to the circadian system by acting at presynaptic 5-HT1B receptors located on retinal axons in the SCN.
Figures


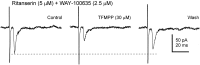




Similar articles
-
Serotonergic modulation of retinal input to the mouse suprachiasmatic nucleus mediated by 5-HT1B and 5-HT7 receptors.J Biol Rhythms. 2001 Feb;16(1):25-38. doi: 10.1177/074873040101600104. J Biol Rhythms. 2001. PMID: 11220775
-
5-HT1B receptor-mediated presynaptic inhibition of GABA release in the suprachiasmatic nucleus.J Neurophysiol. 2005 Jun;93(6):3157-64. doi: 10.1152/jn.00770.2004. Epub 2005 Feb 16. J Neurophysiol. 2005. PMID: 15716370
-
TFMPP, a 5HT1B receptor agonist, inhibits light-induced phase shifts of the circadian activity rhythm and c-Fos expression in the mouse suprachiasmatic nucleus.Neurosci Lett. 1997 Aug 8;231(2):95-8. doi: 10.1016/s0304-3940(97)00534-x. Neurosci Lett. 1997. PMID: 9291149
-
Serotonergic innervation of the hypothalamic suprachiasmatic nucleus and photic regulation of circadian rhythms.Biol Cell. 1997 Nov;89(8):513-23. doi: 10.1016/s0248-4900(98)80007-5. Biol Cell. 1997. PMID: 9618901 Review.
-
Photic entrainment of circadian rhythms in rodents.Chronobiol Int. 1998 Sep;15(5):395-423. doi: 10.3109/07420529808998699. Chronobiol Int. 1998. PMID: 9787933 Review.
Cited by
-
Altered entrainment to the day/night cycle attenuates the daily rise in circulating corticosterone in the mouse.PLoS One. 2014 Nov 3;9(11):e111944. doi: 10.1371/journal.pone.0111944. eCollection 2014. PLoS One. 2014. PMID: 25365210 Free PMC article.
-
Activity-dependent presynaptic effect of serotonin 1B receptors on the somatosensory thalamocortical transmission in neonatal mice.J Neurosci. 2002 Feb 1;22(3):886-900. doi: 10.1523/JNEUROSCI.22-03-00886.2002. J Neurosci. 2002. PMID: 11826118 Free PMC article.
-
Acute ethanol impairs photic and nonphotic circadian phase resetting in the Syrian hamster.Am J Physiol Regul Integr Comp Physiol. 2009 Feb;296(2):R411-8. doi: 10.1152/ajpregu.90782.2008. Epub 2008 Dec 10. Am J Physiol Regul Integr Comp Physiol. 2009. PMID: 19073899 Free PMC article.
-
Cannabinoids excite circadian clock neurons.J Neurosci. 2010 Jul 28;30(30):10061-6. doi: 10.1523/JNEUROSCI.5838-09.2010. J Neurosci. 2010. PMID: 20668190 Free PMC article.
-
Dorsal raphe nucleus-hippocampus serotonergic circuit underlies the depressive and cognitive impairments in 5×FAD male mice.Transl Neurodegener. 2024 Jul 24;13(1):34. doi: 10.1186/s40035-024-00425-w. Transl Neurodegener. 2024. PMID: 39044270 Free PMC article.
References
-
- Azmitia EC, Segal M. An autoradiographic analysis of differential ascending projections of the dorsal and median raphe nuclei of the rat. J Comp Neurol. 1978;179:641–668. - PubMed
-
- Belenky M, Wagner S, Yarom Y, Matzner H, Cohen S, Castel M. The suprachiasmatic nucleus in stationary organotypic culture. Neuroscience. 1996;70:127–143. - PubMed
-
- Belenky M, Sollars PJ, Pickard GE. Electron microscopic immunocytochemical localization of 5-HT1B receptors in the mouse suprachiasmatic nucleus. Soc Neurosci Abstr. 1998;28:1919.
-
- Block M, Zucker I. Circadian rhythms of rat locomotor activity after lesions of the midbrain raphe nuclei. J Comp Physiol [A] 1976;109:235–247.
-
- Boschert U, Amara DA, Segu L, Hen R. The mouse 5-hydroxytryptamine1B receptor is localized predominately on axon terminals. Neuroscience. 1994;58:167–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
