Reactivation of hippocampal cell assemblies: effects of behavioral state, experience, and EEG dynamics
- PMID: 10234037
- PMCID: PMC6782694
- DOI: 10.1523/JNEUROSCI.19-10-04090.1999
Reactivation of hippocampal cell assemblies: effects of behavioral state, experience, and EEG dynamics
Abstract
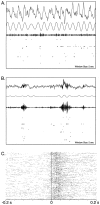
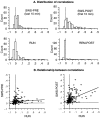
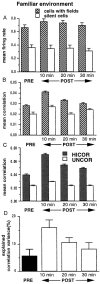
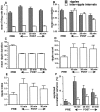
During slow wave sleep (SWS), traces of neuronal activity patterns from preceding behavior can be observed in rat hippocampus and neocortex. The spontaneous reactivation of these patterns is manifested as the reinstatement of the distribution of pairwise firing-rate correlations within a population of simultaneously recorded neurons. The effects of behavioral state [quiet wakefulness, SWS, and rapid eye movement (REM)], interactions between two successive spatial experiences, and global modulation during 200 Hz electroencephalographic (EEG) "ripples" on pattern reinstatement were studied in CA1 pyramidal cell population recordings. Pairwise firing-rate correlations during often repeated experiences accounted for a significant proportion of the variance in these interactions in subsequent SWS or quiet wakefulness and, to a lesser degree, during SWS before the experience on a given day. The latter effect was absent for novel experiences, suggesting that a persistent memory trace develops with experience. Pattern reinstatement was strongest during sharp wave-ripple oscillations, suggesting that these events may reflect system convergence onto attractor states corresponding to previous experiences. When two different experiences occurred in succession, the statistically independent effects of both were evident in subsequent SWS. Thus, the patterns of neural activity reemerge spontaneously, and in an interleaved manner, and do not necessarily reflect persistence of an active memory (i.e., reverberation). Firing-rate correlations during REM sleep were not related to the preceding familiar experience, possibly as a consequence of trace decay during the intervening SWS. REM episodes also did not detectably influence the correlation structure in subsequent SWS, suggesting a lack of strengthening of memory traces during REM sleep, at least in the case of familiar experiences.
Figures
Similar articles
-
Hippocampal memory consolidation during sleep: a comparison of mammals and birds.Biol Rev Camb Philos Soc. 2011 Aug;86(3):658-91. doi: 10.1111/j.1469-185X.2010.00165.x. Epub 2010 Nov 11. Biol Rev Camb Philos Soc. 2011. PMID: 21070585 Free PMC article.
-
Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep.Neuron. 2001 Jan;29(1):145-56. doi: 10.1016/s0896-6273(01)00186-6. Neuron. 2001. PMID: 11182087
-
Cell-Type-Specific Dynamics of Calcium Activity in Cortical Circuits over the Course of Slow-Wave Sleep and Rapid Eye Movement Sleep.J Neurosci. 2021 May 12;41(19):4212-4222. doi: 10.1523/JNEUROSCI.1957-20.2021. Epub 2021 Apr 8. J Neurosci. 2021. PMID: 33833082 Free PMC article.
-
Slow-wave sleep and the consolidation of long-term memory.World J Biol Psychiatry. 2010 Jun;11 Suppl 1:16-21. doi: 10.3109/15622971003637637. World J Biol Psychiatry. 2010. PMID: 20509828 Review.
-
Memory trace reactivation in hippocampal and neocortical neuronal ensembles.Curr Opin Neurobiol. 2000 Apr;10(2):180-6. doi: 10.1016/s0959-4388(00)00079-9. Curr Opin Neurobiol. 2000. PMID: 10753801 Review.
Cited by
-
Contextual memory engrams, and the neuromodulatory influence of the locus coeruleus.Front Mol Neurosci. 2024 Feb 5;17:1342622. doi: 10.3389/fnmol.2024.1342622. eCollection 2024. Front Mol Neurosci. 2024. PMID: 38375501 Free PMC article. Review.
-
The Neuroscience of Spatial Navigation and the Relationship to Artificial Intelligence.Front Comput Neurosci. 2020 Jul 28;14:63. doi: 10.3389/fncom.2020.00063. eCollection 2020. Front Comput Neurosci. 2020. PMID: 32848684 Free PMC article. Review.
-
The relationship between gamma frequency and running speed differs for slow and fast gamma rhythms in freely behaving rats.Hippocampus. 2015 Aug;25(8):924-38. doi: 10.1002/hipo.22415. Epub 2015 Jan 20. Hippocampus. 2015. PMID: 25601003 Free PMC article.
-
Lempel-Ziv complexity of cortical activity during sleep and waking in rats.J Neurophysiol. 2015 Apr 1;113(7):2742-52. doi: 10.1152/jn.00575.2014. Epub 2015 Feb 25. J Neurophysiol. 2015. PMID: 25717159 Free PMC article.
-
Hippocampal network oscillations at the interplay between innate anxiety and learned fear.Psychopharmacology (Berl). 2019 Jan;236(1):321-338. doi: 10.1007/s00213-018-5109-z. Epub 2018 Nov 11. Psychopharmacology (Berl). 2019. PMID: 30417233 Review.
References
-
- Bloch V, Hennevin E, Leconte P. Relationship between paradoxical sleep and memory processes. In: Brazier MAB, editor. Brain mechanisms in memory and learning: from the single neuron to man. Raven; New York: 1979. pp. 329–343.
-
- Buzsáki G. Hippocampal sharp waves: their origin and significance. Brain Res. 1986;398:242–252. - PubMed
-
- Buzsáki G. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience. 1989;31:551–570. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
