Intrinsic neurons of fastigial nucleus mediate neurogenic neuroprotection against excitotoxic and ischemic neuronal injury in rat
- PMID: 10234042
- PMCID: PMC6782716
- DOI: 10.1523/JNEUROSCI.19-10-04142.1999
Intrinsic neurons of fastigial nucleus mediate neurogenic neuroprotection against excitotoxic and ischemic neuronal injury in rat
Abstract
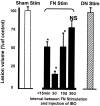
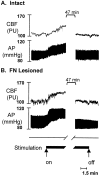
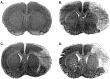
Electrical stimulation of the cerebellar fastigial nucleus (FN) elevates regional cerebral blood flow (rCBF) and arterial pressure (AP) and provides long-lasting protection against focal and global ischemic infarctions. We investigated which neuronal element in FN, perikarya or axons, mediates this central neurogenic neuroprotection and whether it also protects against excitotoxicity. In anesthetized rats, the FN was stimulated for 1 hr, and ibotenic acid (IBO) was microinjected unilaterally into the striatum. In unstimulated controls, the excitotoxic lesions averaged approximately 40 mm3. Stimulation of FN, but not dentate nucleus (DN), significantly reduced lesion volumes up to 80% when IBO was injected 15 min, 72 hr, or 10 d, but not 30 d, thereafter. In other rats, intrinsic neurons of FN or DN were destroyed by pretreatment with IBO. Five days later, the FN was stimulated, and 72 hr later, IBO was microinjected into the striatum. Lesions of FN, but not DN, abolished neuroprotection but not the elevations of rCBF and AP elicited from FN stimulation. Excitotoxic lesions of FN, but not DN, also abolished the 37% reduction in focal ischemic infarctions produced by middle cerebral artery occlusion. Excitation of intrinsic FN neurons provides long-lasting, substantial, and reversible protection of central neurons from excitotoxicity, as well as focal ischemia, whereas axons in the nucleus, probably collaterals of ramified brainstem neurons, mediate the elevations in rCBF, which do not contribute to neuroprotection. Long-lived protection against a range of injuries is an unrecognized function of FN neurons transmitted over pathways distinct from those regulating rCBF.
Figures






Similar articles
-
Stimulation of the subthalamic vasodilator area and fastigial nucleus independently protects the brain against focal ischemia.Brain Res. 2001 Aug 31;912(1):47-59. doi: 10.1016/s0006-8993(01)02602-6. Brain Res. 2001. PMID: 11520492
-
Global reduction in cerebral blood flow and metabolism elicited from intrinsic neurons of fastigial nucleus.Brain Res. 1989 Oct 23;500(1-2):177-92. doi: 10.1016/0006-8993(89)90312-0. Brain Res. 1989. PMID: 2605490
-
Brief electrical stimulation of cerebellar fastigial nucleus conditions long-lasting salvage from focal cerebral ischemia: conditioned central neurogenic neuroprotection.Brain Res. 1998 Jan 5;780(1):161-5. Brain Res. 1998. PMID: 9497093
-
Autonomic and vasomotor regulation.Int Rev Neurobiol. 1997;41:121-49. doi: 10.1016/s0074-7742(08)60350-5. Int Rev Neurobiol. 1997. PMID: 9378586 Review.
-
Central neurogenic neuroprotection: central neural systems that protect the brain from hypoxia and ischemia.Ann N Y Acad Sci. 1997 Dec 19;835:168-86. doi: 10.1111/j.1749-6632.1997.tb48628.x. Ann N Y Acad Sci. 1997. PMID: 9616772 Review.
Cited by
-
Neurogenic neuroprotection.Cell Mol Neurobiol. 2003 Oct;23(4-5):651-63. doi: 10.1023/a:1025088516742. Cell Mol Neurobiol. 2003. PMID: 14514022 Free PMC article. Review.
-
Electrical stimulation of cerebellar fastigial nucleus: mechanism of neuroprotection and prospects for clinical application against cerebral ischemia.CNS Neurosci Ther. 2014 Aug;20(8):710-6. doi: 10.1111/cns.12288. Epub 2014 Jun 16. CNS Neurosci Ther. 2014. PMID: 24930936 Free PMC article. Review.
-
PRIMED2 Preclinical Evidence Scoring Tool to Assess Readiness for Translation of Neuroprotection Therapies.Transl Stroke Res. 2022 Apr;13(2):222-227. doi: 10.1007/s12975-021-00922-4. Epub 2021 Jul 1. Transl Stroke Res. 2022. PMID: 34196953 Free PMC article.
-
From Molecule to Patient Rehabilitation: The Impact of Transcranial Direct Current Stimulation and Magnetic Stimulation on Stroke-A Narrative Review.Neural Plast. 2023 Feb 28;2023:5044065. doi: 10.1155/2023/5044065. eCollection 2023. Neural Plast. 2023. PMID: 36895285 Free PMC article. Review.
-
Neurogenic neuroprotection: clinical perspectives.Funct Neurol. 2012 Oct-Dec;27(4):207-16. Funct Neurol. 2012. PMID: 23597434 Free PMC article.
References
-
- Baron JC, Rougemont D, Soussaline F, Bustany P, Crouzel C, Bousser MG, Comar D. Local interrelationships of cerebral oxygen consumption and glucose utilization in normal subjects and in ischemic stroke patients: a positron tomography study. J Cereb Blood Flow Metab. 1984;4:140–149. - PubMed
-
- Batton RR, III, Jayaraman A, Ruggiero DA, Carpenter MB. Fastigial efferent projections in the monkey: an autoradiographic study. J Comp Neurol. 1977;174:281–306. - PubMed
-
- Beirne JP, Pearlstein RD, Massey GW, Warner DS. Effect of halothane in cortical cell cultures exposed to N-methyl-d-aspartate. Neurochem Res. 1998;23:17–23. - PubMed
-
- Bidman H-J, Oermann E, Schleicher A, Kato K, Kinscherf R, Buchkremer-Ratzmann I, Witte OW, Zilles K. Copper-zinc superoxide dismutase and isolectin B4 binding are markers for associative and transhemispheric diachisis induced by focal ischemia in rat cortex. Neurosci Lett. 1997;228:163–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
